20.1 Experimental gas laws
1/13
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
14 Terms
pressure
force per unit area
Boyle’s Law
pV= constant
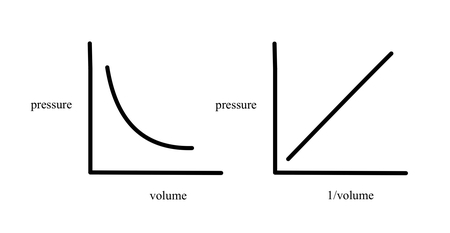
Explain Boyle’s Law
The pressure of a gas is inversely proportional to its volume at constant temperature.
As volume increases, pressure decreases, and vice versa.
Charles’ Law
V/ T= constant
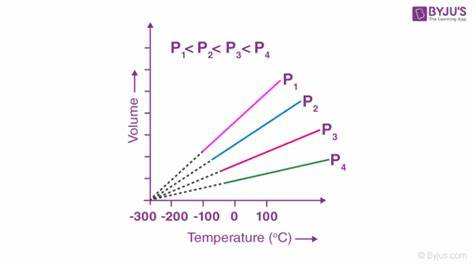
Explain Charles’ Law
The volume of a gas is directly proportional to its temperature at constant pressure. As temperature increases, volume also increases, and vice versa.
What is the volume of a gas at 0 K?
0
isobaric change
A change at constant pressure is called an __________ change
When work is done to change the volume of a gas, energy must be transferred by __________ to keep the pressure ________________.
heating
constant
Equation for work done by a piston
p∆V
Pressure Law
p/T= constant
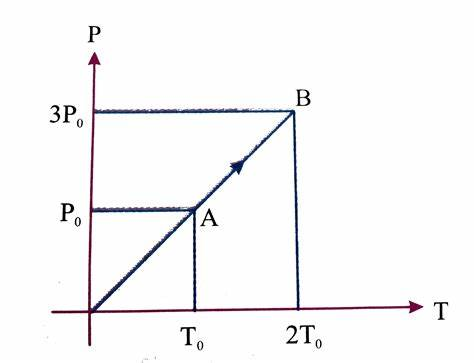
Explain the pressure law
The pressure of a gas is directly proportional to its absolute temperature when the volume remains constant.
What causes breathing difficulties for underwater swimmers at significant depths?
The extra pressure from the water above them compresses gases in the lungs, leading to breathing difficulties.
What is 'the bends' and why is it dangerous for divers?
'The bends' is a life-threatening condition caused by the rapid release of dissolved nitrogen from the blood if a diver ascends too quickly.