Atomic and nuclear physics
1/28
Earn XP
Description and Tags
Physics internal level 2
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
29 Terms
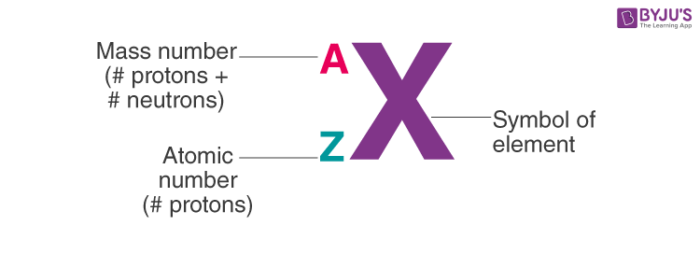
Which number goes on top?
mass number
What is the reason for a vaccume chamber
The alpha particle (He²^+) removes electrons from neutral particles in the air to become a neutral He atom and therefore ionising the air.
What is the equation for He²^+ particles ionising the air?
He²^+ + 2e^- » He
What is the reason for zinc sulfide on the screen?
The alpha particles hitting the screen causes flashes of light which can be detected.
what is the reason for beam of alpha particles?
So the alpha particles travel toward gold foil in straight line.
What is the reason for circular screen?
To check for deflecctions of alpha particles through full 360 degree.
Why did most of the aplha particles go straight through?
Most of the atom is empty space
There is a very small and dence nucleus.
Why were some of the alpha particles deflected through large angles?
The positive alpha particle was close enough to the gold nucleus to experience a force to repel it.
Why were some of the alpha particles reflected straight back?
The positive alpha particle was almost in line with a positive nucleus and the repulsive force between the two positive charges caused a dramatic change in direction.
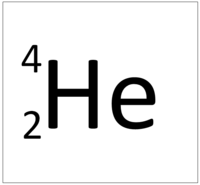
Which ray consists of 2 protons and 2 neutrons?
Alpha ray.
beta has ___ protons
0
Beta is also known as?
An electron.
Which ray has medium ionizing ability?
Beta ray.
Gamma rays have ___ protons and ___ neutrons.
0
Which rays have no charge or mass?
Gamma rays have no charge or mass.
Which rays have highest penentrating power therefore highest energy?
Gamma rays have highest penentrating power and therefore have the highest energy.
Which rays are electromagnetic waves?
Gamma rays are electomagnetic waves.
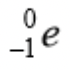
Which ray is an electron particle?
Beta ray.
How is the electron particle formed? What is this equation?
A neutron splits into an electron and a proton.
1,0 n » 1,1 p + 0,-1 e
What is the charge of a Beta particle?
-1
Which feilds can be alpha particles be detected by?
Alpha particles can be detected by photographic film, electric and magnetic feilds.
During beta ray formation, what happens to the electron and proton?
The proton remains in the nucleus and the electron is emitted as a Beta particle.
Which feilds can Beta particles be detected by?
Beta rays can be detected by photographic film, electric and magnetic feilds.
Which feilds are Gamma rays detected by?
Gamma rays are only detected by photographic film.
Which ray has low ionizing ability?
Gamma rays have low ionizing ability.
What is an isotope?
An isotope is an element with the same number of protons but different numbers of neutrons in the nucleus of the atom, therefore having a different mass.
What is an Alpha particle?
An alpha particle is a helium nucleus consisting of 2 protons and 2 neutrons.
How are Gamma rays formed ?
Gamma rays form from the energy released when unstable nuclei become stable (gamma decay)
How is an alpha ray formed?
An alpha ray is formed when an unstable atomic nucleus undergoes alpha decay, emitting an alpha particles consisting of 2 protons and 2 neutrons