lec 7 - water movement
1/23
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
24 Terms
where water is gained or lost from
always from ECF irst - transitional compartment
total body water
adult male ~60%
adult female ~55%
of body weight
total body water distributions
ECF = 33% of TBW
ICF = 67% of TBW
interstitial fluid = 75% ECF
plasma 20% ECF
transcellular fluid 5% ECF
osmotic equilibrium
water moves freely between the ICF and ECF comparments
the volume and composition of body fluid compartments are highly regulated homeostatic variables
deviation in fluid homeostasis results in illness and may be life-threatening
net diffusion of water across a membrane
diffusional movement of water from a region of higher water concentratio to an area of lower concentration
change in water concentration due to difference in solute concentration
driving force for water movement
passive movement
cell membranes cannot move water against a gradient
driving force is the difference in osmotic pressure across the membrane
in certain tissues (e.g. capillaries, glomerulus) hydrostatic pressures also influences water movement (not for cells)
osmotic pressure equation (JH2O)
JH2O = Lp (Δπ - ΔP)
Lp = hydraulic conductivity (how easily does water cross membrane)
Δπ = osmotic pressure difference
ΔP = hydrostatic pressure difference (not for cells)
osmotic pressure
the pulling force acting on water
water moves by osmosis down its concentration gradient - where there are low solutes/a lot of water -> where there are many solutes/less water
osmosis continues until the solute/water concentrations in both compartments are equal
measuring osmotic pressure
force is applied to exactly oppose osmosis
force is applied to compartment B (hydrostatic pressure) until osmosis stops
means of quantitatively measuring the osmotic driving force in an artificial system
animal cell membranes cant withstand hydrostatic pressure gradients, so dont measure osmotic pressure experimentally
osmotic pressure - van't Hoff equation
π = RTCi
π = osmotic pressure (Pa)
R = gas constant (8.314 JK-1mol-1)
T = temperature in K (310.15K/37 degrees in biological systems)
C = total solute concentration (molL-1)
i = number of solute molecules formed by dissociation
osmolarity - calculation
molarity (molL-1) x particles formed from dissociation = osmolarity (osmolL-1)
so a particle that dissociates e.g. NaCl has to be multiplied by 2 becuase it disociates into 2 ions, but things that don't dissociate are multiplied by 1
hyposmotic solutions
<275 mOsmL-1
fewer solute molecules
lower osmorality
higher water concentration
isosmotic solutions
~300mOsmL-1
similar number of solute molecules
similar osmolarity
similar water concentration
hyperosmotic solutions
>310 mOsmL-1
more solute molecules
higher osmolarity
lower water concentration
diffusion of water across the lipid bilayer
water is a small molecule so can directly diffuse through lipid bilayer
relatively slow (low hydraulic conductivity) and unregulated
all biological membranes are somewhat permeable to water, but some cell membranes (e.g. red blood cells, proximal renal tubule) have very high hydraulic conductivities
aquaporins increase the conductivity of the membrane
aquaporins
non-gated tunnel
small membrane bound water pores
100 fold increase in hydaulic conductivity - highly selective for water over ions
selectivity determined by asparagine residues (prevents ion passage)
AQP2 (in distal nephron) is under hormonal control
difference in osmotic pressure still provides the driving force
tonicity - what
describes the volume change of a cell
isotonic solution
if the cell in the solution does not change size/volume at equilibrium = the solution is isotonic
no net movement of water -> no change in cell size
hypertonic solution
if the cell loses water and shrinks at equilibrium then the solution is hypertonic
net movement of water out of the cells -> cells shrink
osmolarity vs tonicity
osmolarity:
either solution or the cell itself
chemical measure of solutes in solution
canbe calculated or measured (mOsmL-1)
tonicity:
describes only the solution
experimentally determined - what does the solution do to cell volume
determined by osmolarity and solute permeability i.e. whether the solute in solution can enter the cell
IV solutions
the tonicity not the osmolarity is what is important
0.9% NaCl
5% glucose
both isosmotic
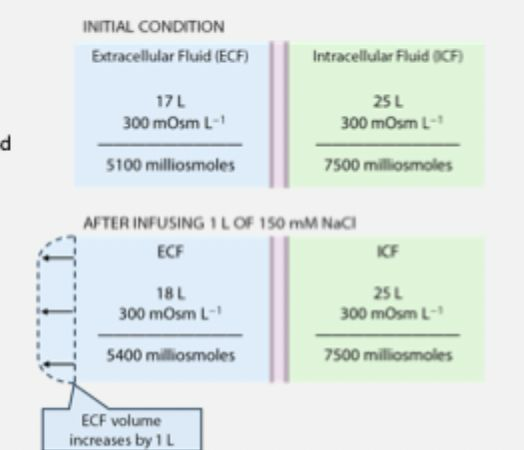
IV solutions - NaCl
isosmotic 150mmolL-1 NaCl is isotonic (Na+ and Cl- are both non-penetrating solutes)
IV solutions - glucose
at equilibrium in a healthy person IV osmostic glucose is effectively hypotonic
in isolated red blood cells, glucose is considered a non-penetrating solute
omslarity vs tonicity
