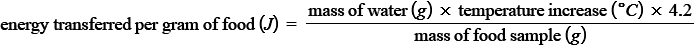2b. Structure & Function in Living Organisms: Biological molecules + Movement of substances into and out of cells + Nutrition
1/66
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
67 Terms
what are the three main categories of biological molecules, and what elements do they contain?
carbohydrate: carbon, oxygen, hydrogen
protein: carbon, oxygen, hydrogen (some contain small amounts of other elements like nitrogen and sulfur)
lipid: carbon, oxygen, hydrogen
what are carbohydrates made up of? what types are there?
they can be small, simple sugars, or more complex larger molecules
monosaccharides are simple sugars:
glucose (C6H12O6), fructose and galactose
disaccharides:
maltose: glucose + glucose
sucrose: glucose + fructose
lactose: glucose + galactose
polysaccharides:
starch: in plants
glycogen: in the liver
cellulose: in cell walls
all are made of many glucose molecules
polysaccharides are insoluble and therefore useful as storage molecules
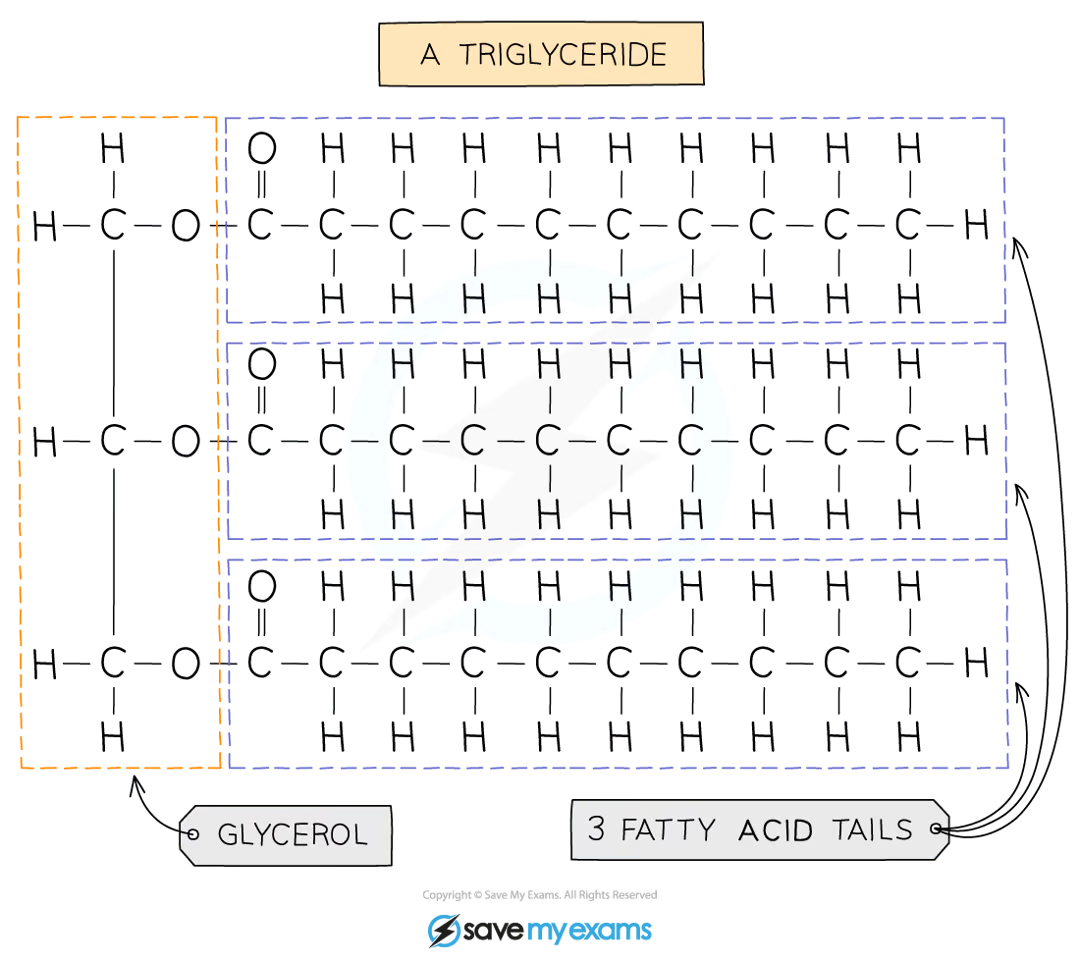
what are lipids made up of? what categories are they divided into?
triglycerides
glycerol + 3 fatty acids
they are divided into fats (solid at room temperature) and oils (liquids at room temperature)
what are proteins made up of? what do they do? what are some examples?
long chains of amino acids
there are hundreds of thousands of different proteins
they have different shapes, and their shape determines their function
examples include enzymes, haemoglobin, ligaments, keratin
how would you prepare a food sample for a food test?
break up the food using a pestle and mortar
transfer to a test tube and add distilled water
mix the food with the water
filter the mixture using a funnel and filter paper
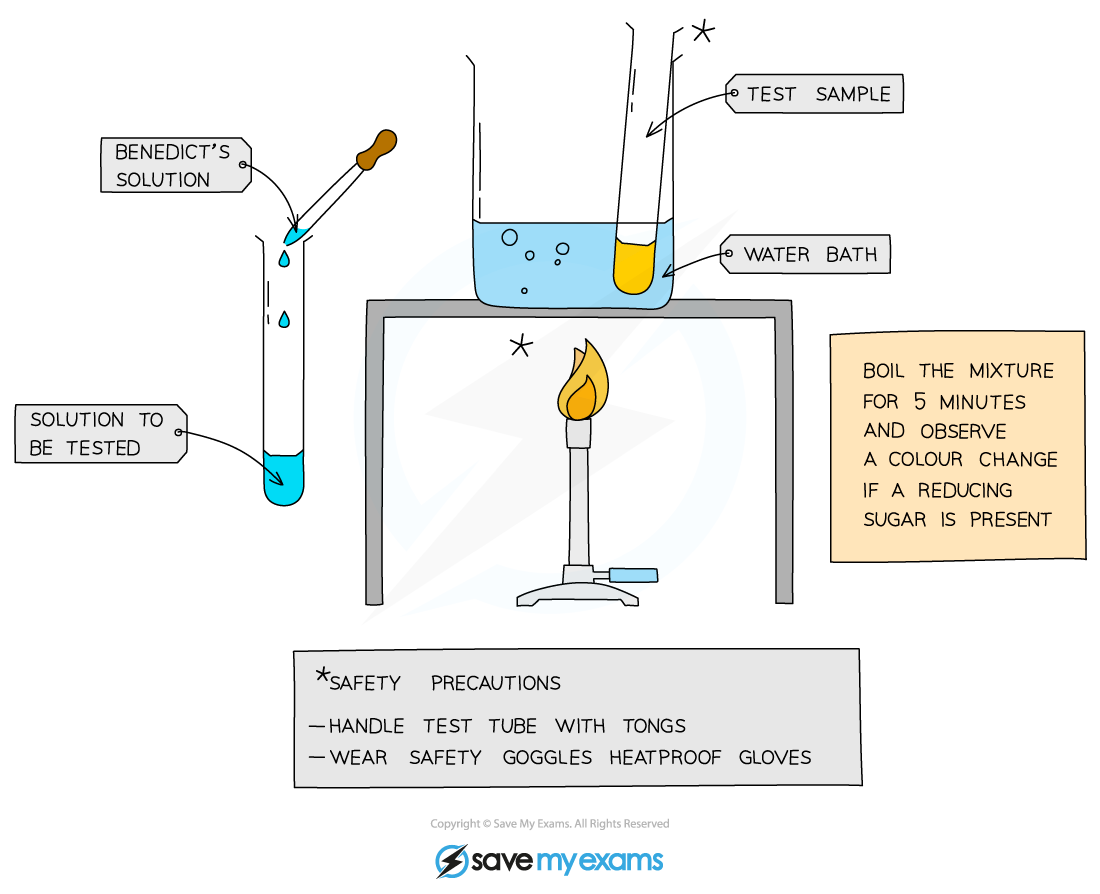
what is the test for glucose?
add Benedict’s solution to a sample solution in a test tube
heat in a water bath for 5 minutes
take the test tube out of the bath and observe the colour
positive test shows a colour change from blue to brick red
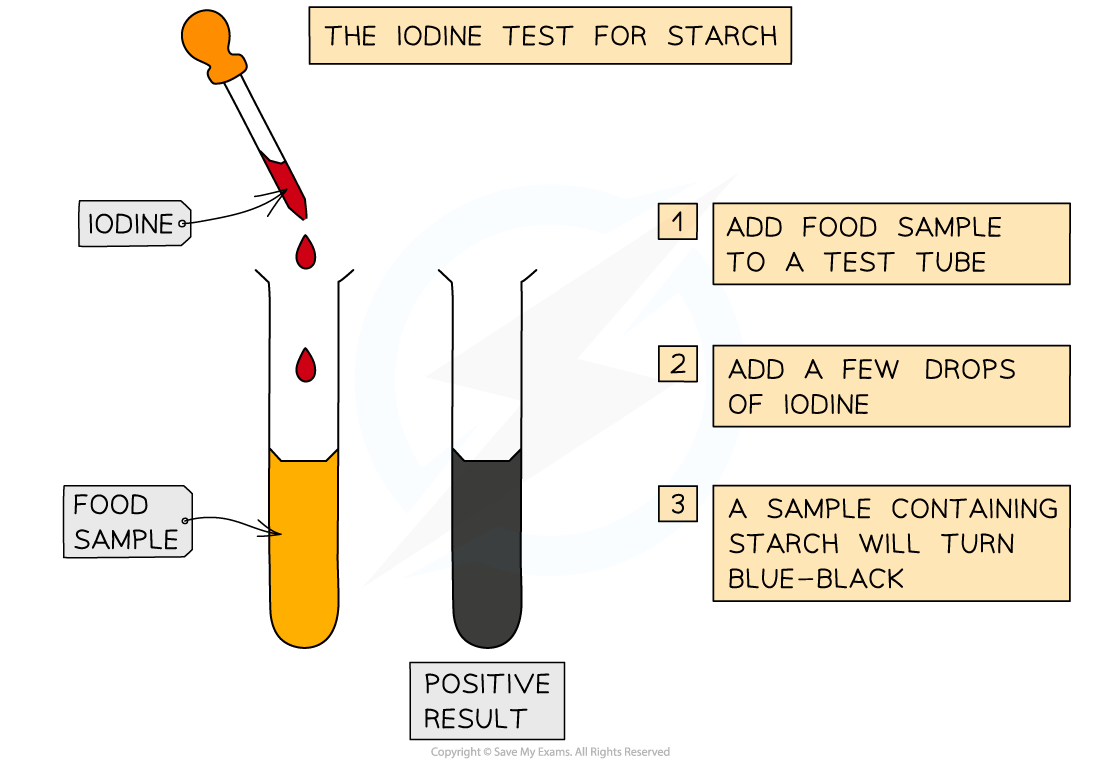
what is the test for starch?
add drops of iodine solution to the sample
a positive test will show a colour change from orange-brown to blue-black
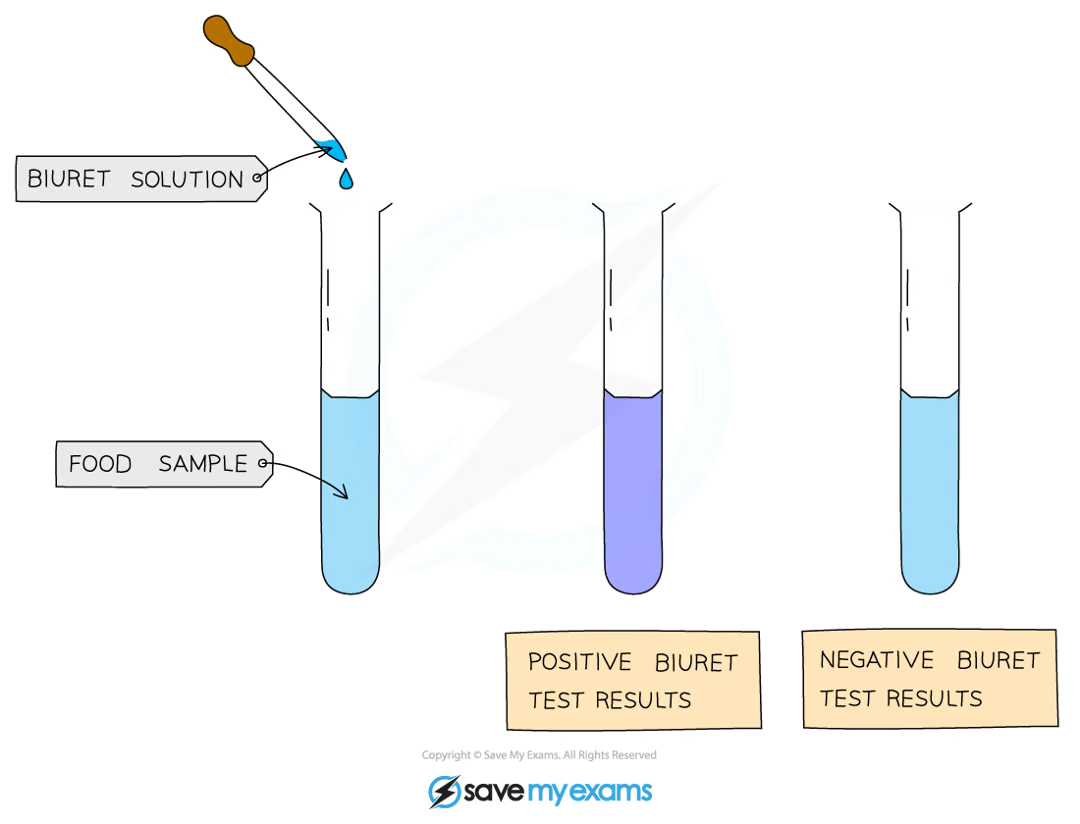
what is the test for protein?
add drops of Biuret solution to the sample
a positive test will show a colour change from blue to violet/purple
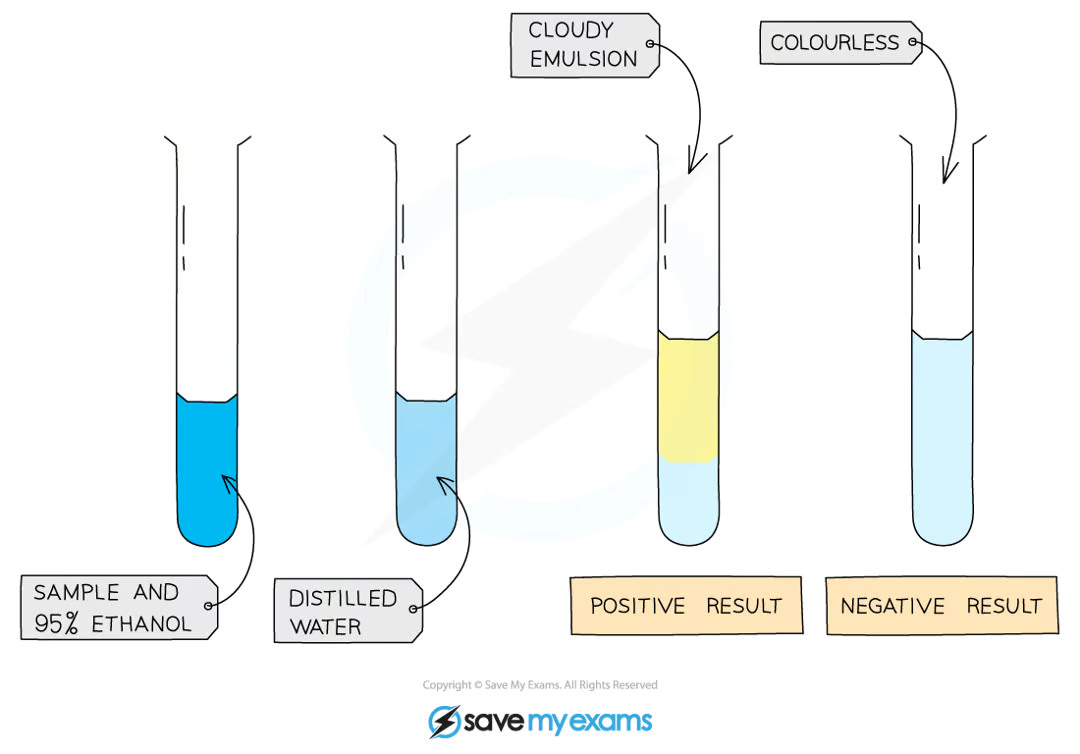
what is the test for lipids (fats)?
mix the food sample with 4cm3 of ethanol and shake
allow time for the sample to dissolve
strain the ethanol solution into another test tube
add the solution to an equal volume of cold distilled water
a positive test will show a cloudy emulsion forming
what are enzymes? why are they necessary to all living organisms?
proteins that act as biological catalysts to speed up the rate of a chemical reaction without being changed or used up
they are necessary because they maintain reaction speeds of all metabolic reactions at a rate that can sustain life
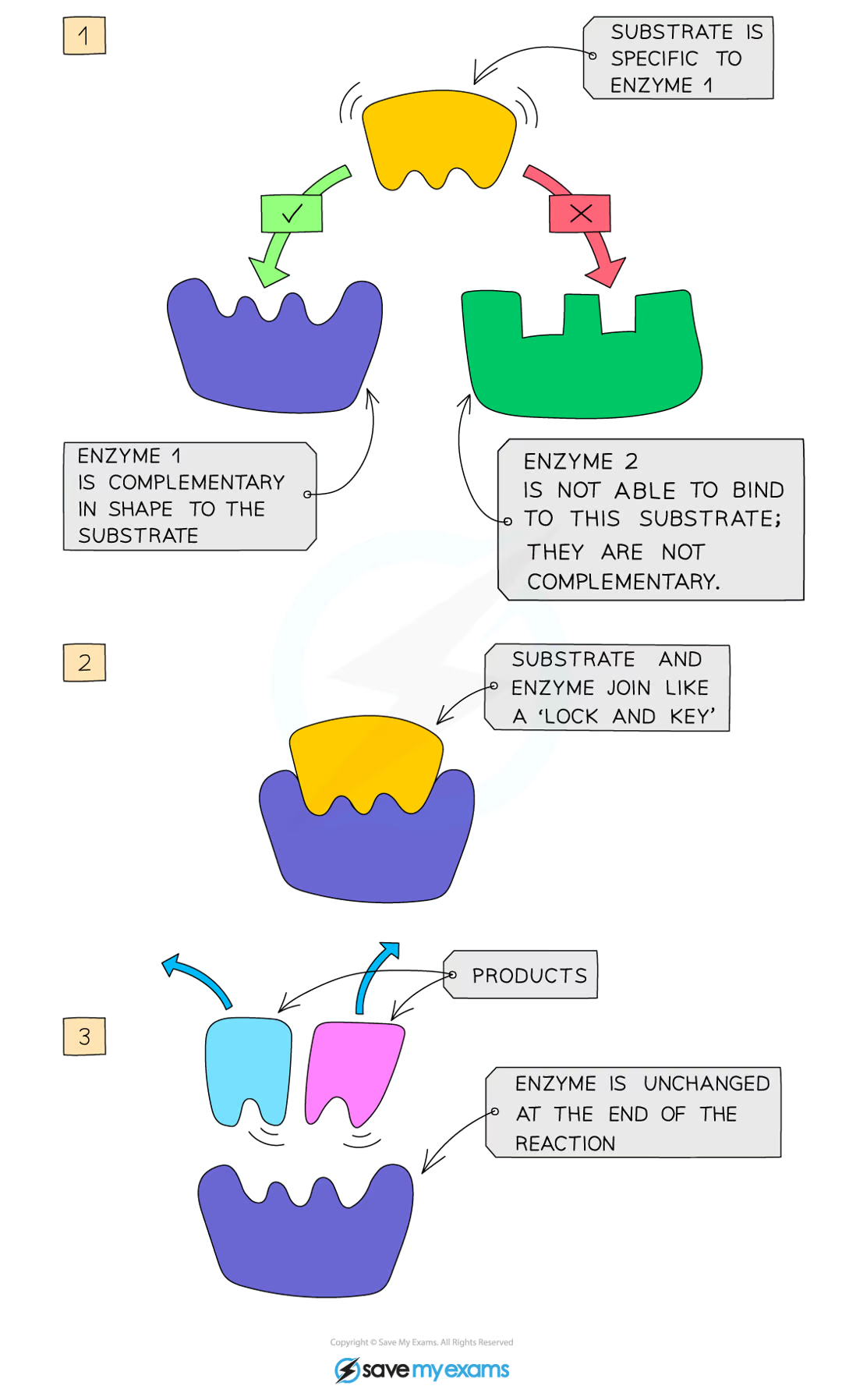
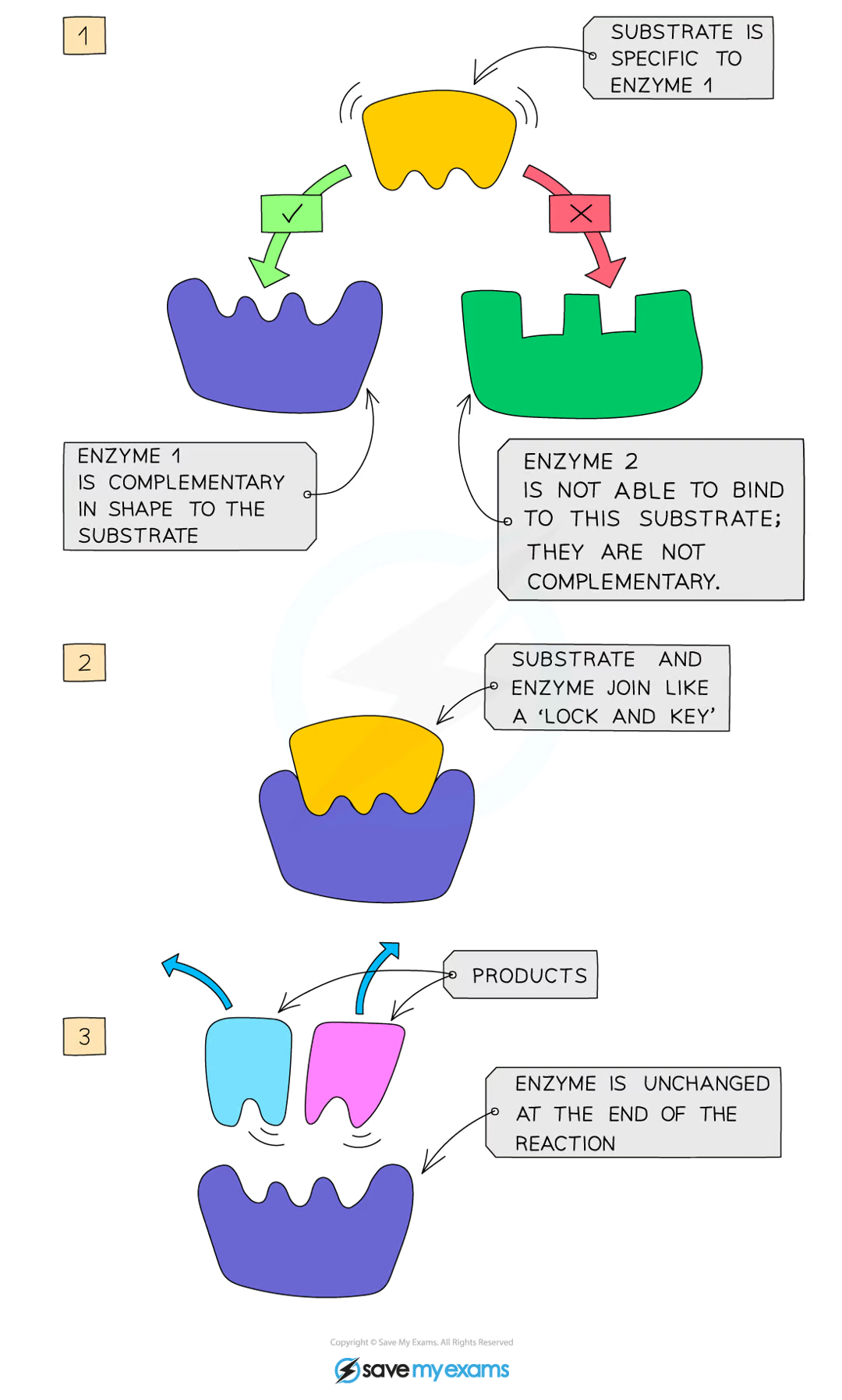
how do enzymes work?
enzymes are specific to one particular substrate, as the active site of the enzyme is a complementary shape to the substrate
the substrate slots into the enzyme’s active site, and together they form the enzyme-substrate complex
the enzyme catalyses the reaction and the products leave the active site as they no longer fit, and the enzyme is free to take up another substrate
what is an enzyme’s ‘optimum temperature’? what is it in the human body?
the temperature enzymes work fastest at is their ‘optimum temperature’
in the human body, it is 37⁰C
what happens when an enzyme is heated to a temperature beyond the optimum temperature?
the peptide bonds that hold the enzyme together break and the enzyme changes shape - this is known as denaturation
when the active site changes shape, the substrate can no longer fit into it and the enzyme can no longer catalyse the reaction
denaturation is irreversible
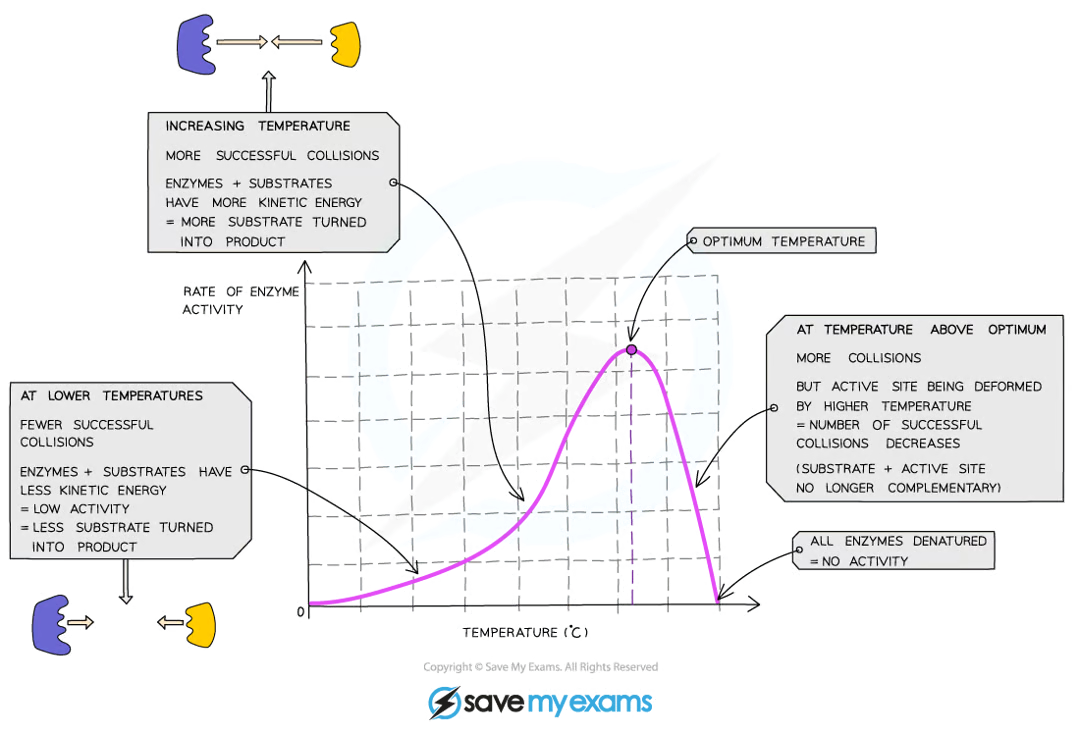
how does temperature affect enzyme’s productivity?
increasing the temperature towards the optimum increases the activity of enzymes as they have more thermal energy, and therefore more kinetic energy, so the molecules move faster and the number of collisions with substrate molecules increases, leading to a faster rate of reaction
low temperatures do not denature enzymes - they just make them work more slowly due to a lack of kinetic energy
PRACTICAL: Investigating temperature & enzyme activity
METHOD
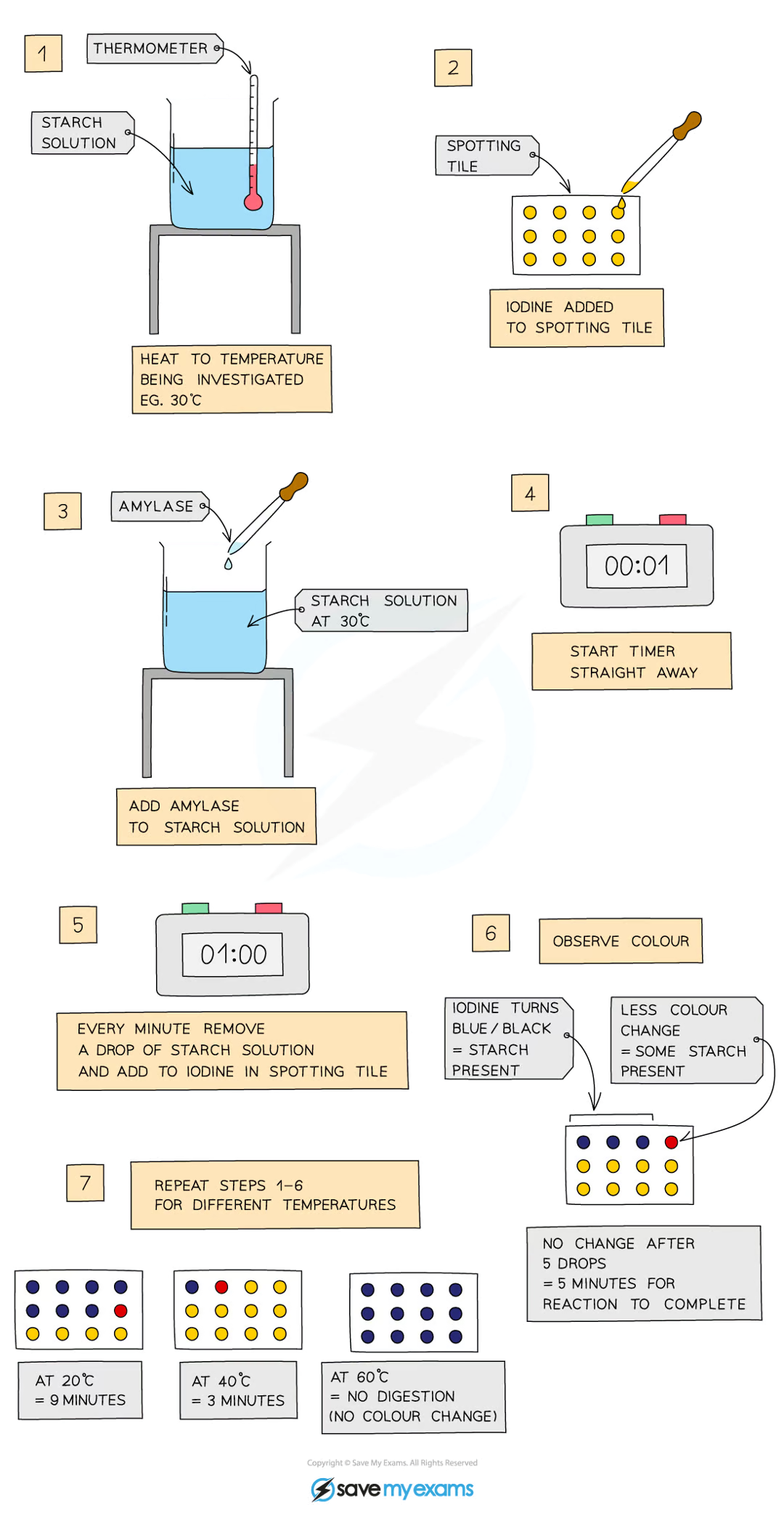
add 5cm³ of starch solution to a test tube and heat to a set temperature using a water bath
add a drop of iodine solution to each of the wells of a spotting tile
use a syringe to add 2cm³ of amylase to the starch solution and mix well
using a stopwatch, every minute, transfer a drop of solution to a new well of iodine solution (initially, the solution should turn blue-black)
repeat this process until the iodine solution stops turning blue-black
record the time taken for the reaction to be completed
repeat the investigation for a range of temperatures (from 20°C to 60°C)
RESULTS
amylase is an enzyme which breaks down starch
the quicker the reaction is completed, the faster the enzyme is working
this investigation shows:
at the optimum temperature, the iodine stopped turning blue-black the fastest
this is because the enzyme is working at its fastest rate
at colder temperatures, the iodine took a longer time to stop turning blue-black
this is because the amylase enzyme is working slowly due to low kinetic energy and few collisions between the amylase and the starch
at hotter temperatures, the iodine turned blue-black throughout the whole investigation
this is because the amylase enzyme was denatured and so could no longer bind with the starch and break it down
PRACTICAL: Investigating temperature & enzyme activity
(CORMMS)
C - change the temperature in each repeat
O - does not apply
R - repeat the investigation several times to ensure results are reliable
M - measure the time taken
M - for the iodine to stop turning black
S - control the concentration and volume of starch solution, iodine, and amylase used
what is the optimum pH for most enzymes?
7
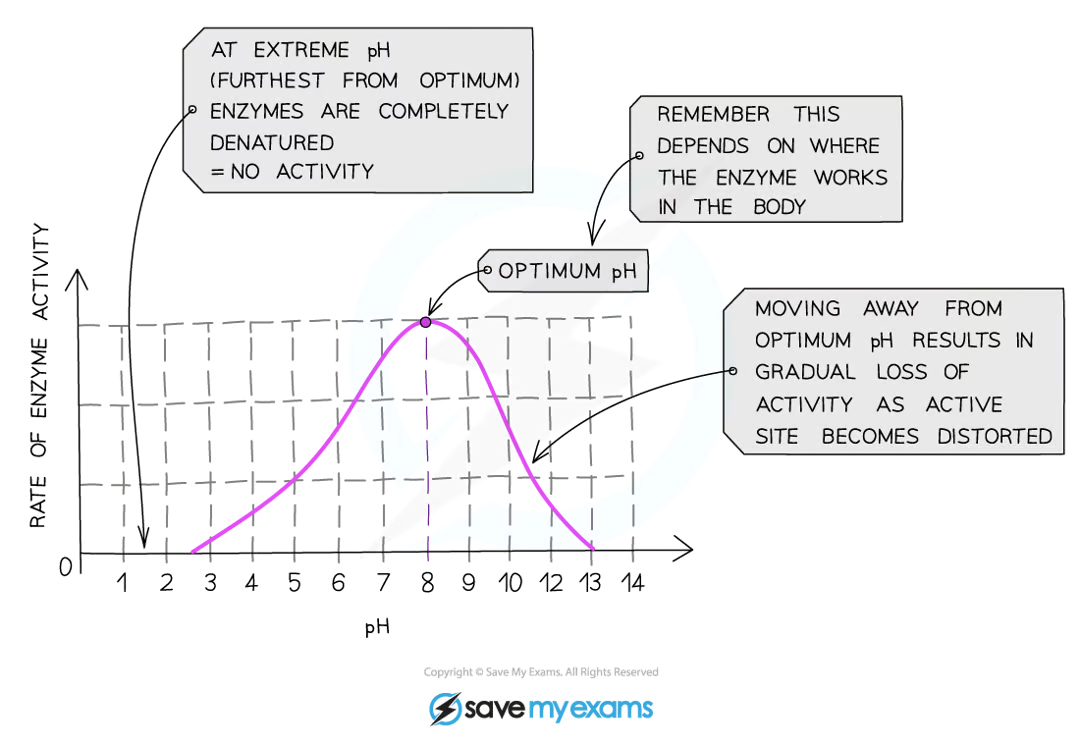
how does pH affect enzymes?
if the pH is too high or too low, the bonds that hold the amino acid chaintogether can be disrupted/destroyed
this changes the shape of the active site, so the substrate can no longer fit into it, reducing the rate of reactivity
moving too far away from the optimum pH will cause the enzyme to denature and activity will stop
PRATICAL: Investigating pH & enzyme activity
METHOD
add a drop of iodine solution to each of the wells of a spotting tile
use a syringe to place 2cm³ of amylase into a test tube
add 1cm³ of buffer solution (at a specific pH) to the test tube
add 2cm³ of starch solution to the amylase and buffer solution. start the stopwatch whilst mixing using a glass rod
every 10 seconds, transfer a droplet of the solution to a new well of iodine solution (which should initially turn blue-black)
repeat this transfer process until the iodine solution stops turning blue-black
record the time taken for the reation to be completed
repeat the investigation with buffers at different pH values
RESULTS
amylase is an enzyme which breaks down starch
when the iodine solution remains orange-brown, all the starch has been digested
this investigation shows:
at the optimum pH, the iodine stopped turning blue-black the fastest
this is because the enzyme is working at its fastest rate
at higher or lower pHs the iodine took a longer time to stop turning blue-black or continued to turn blue-black for the entire investigation
this is because on either side of the optimum pH, the enzymes start to denature and as a result are unable to bind with the starch and break it down
PRATICAL: Investigating pH & enzyme activity
(CORMMS)
C - change the pH of the buffer solution
O - this is not relevant as we are not using an organism
R - repeat the investigation several times to ensure results are reliable
M - measure the time taken
M - for the iodine to stop turning blue-black
S - control the concentration and volume of amylase, iodine and starch solution used in the investigation
define diffusion
the movement of molecules from an area of higher concentration to an area of lower concentration
how do molecules move?
down the concentration gradient
randomly - the result of this movement is the spreading out of molecules until they are at even concentration throughout the available space
how do molecules diffuse in living organisms?
molecules move into or out of cells by diffusion when they cross the cell membrane, which is partially permeable
define osmosis
the movement of free water molecules from an area of higher concentration to an area of lower concentration through a partially permeable membrane
how does osmosis work in animal cells?
without a cell wall, osmosis can have severe effects on animal cells:
in concentrated solutions, cells lose water and become crenated
in distilled water, the cell gains water and bursts as it lacks a cell wall to maintain structure
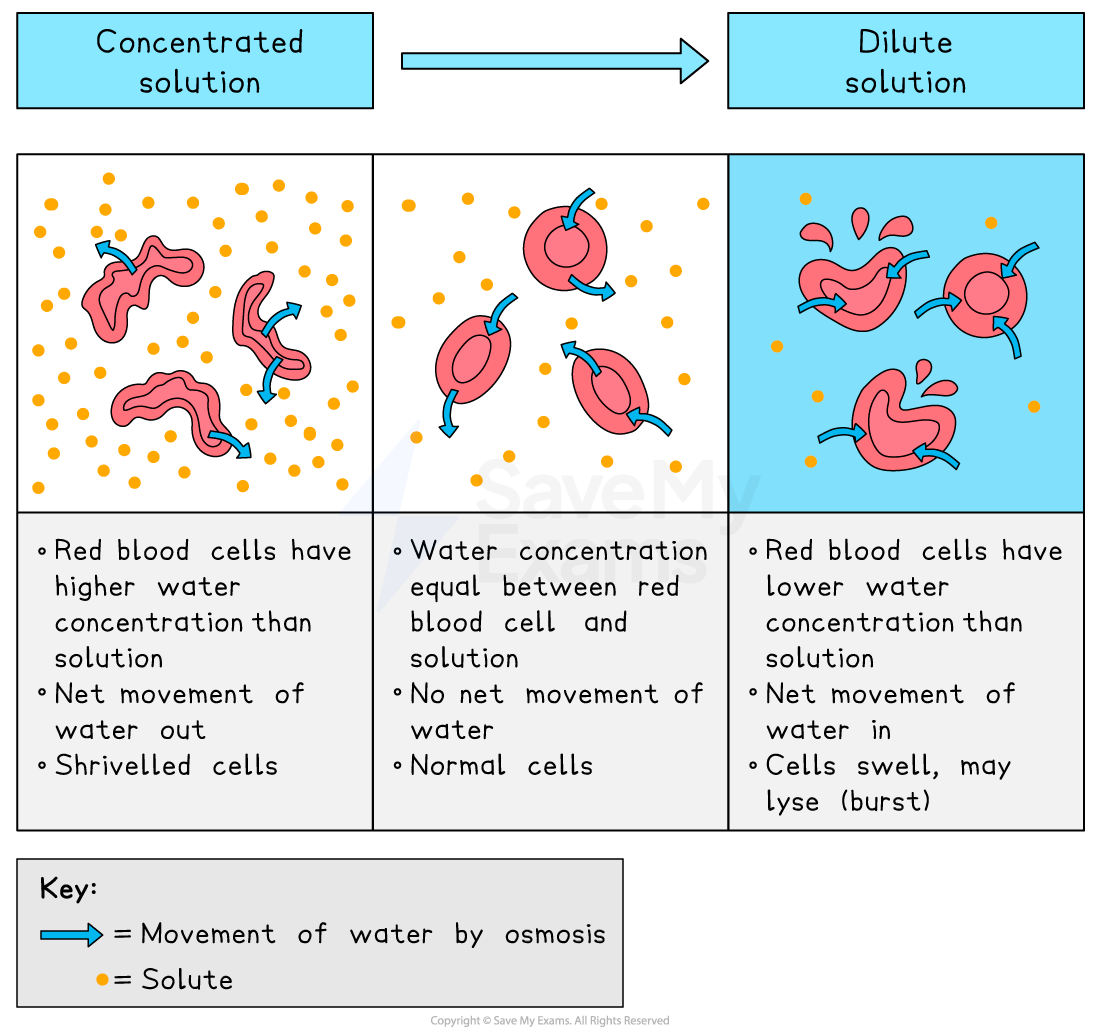
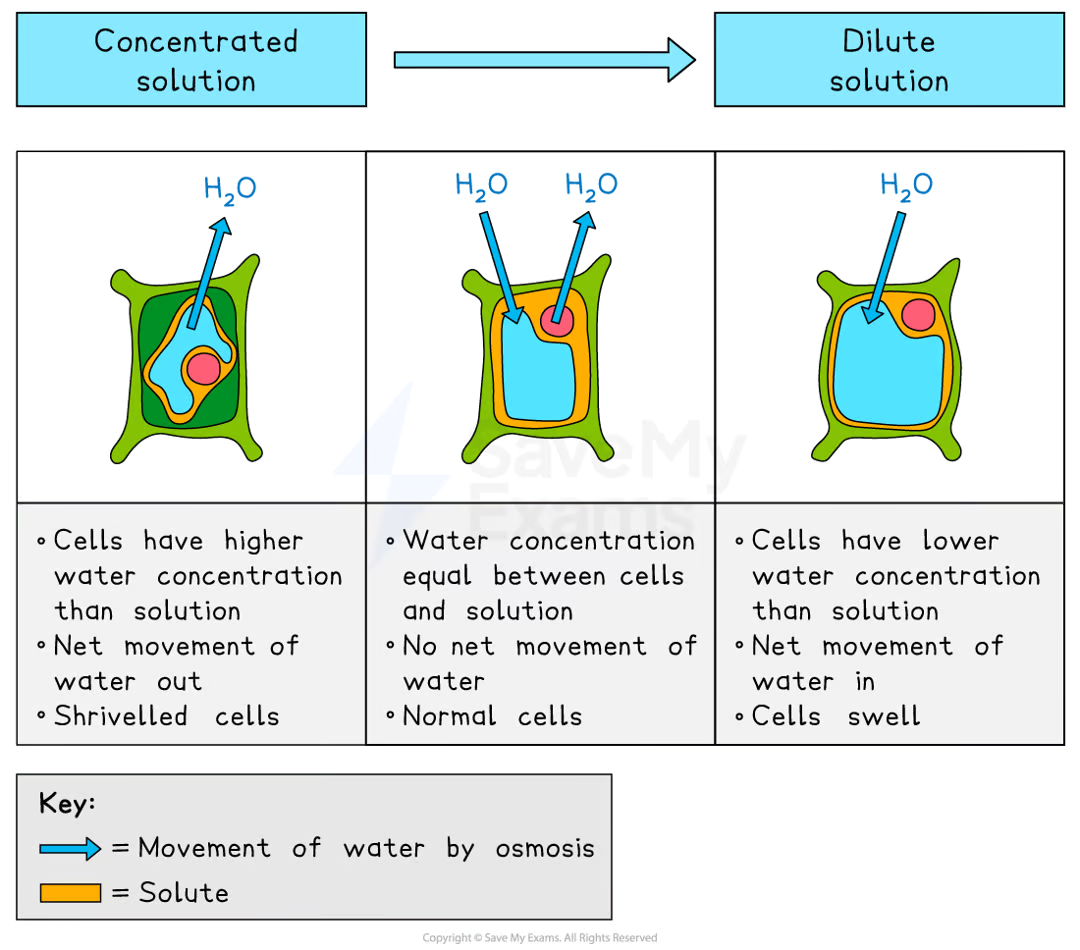
how does osmosis work in plant cells?
due to the cell wall, plant cells are protected from bursting:
in concentrated solutions, the cell loses water, the vacuole shrinks, and the cell membrane pulls away from the wall, making the cell plasmolysed
in distilled water, the cell gains water, the vacuole expands, and the membrane pushes against the cell wall, making the cell turgid
turgid cells provide structural support and prevent wilting in plants
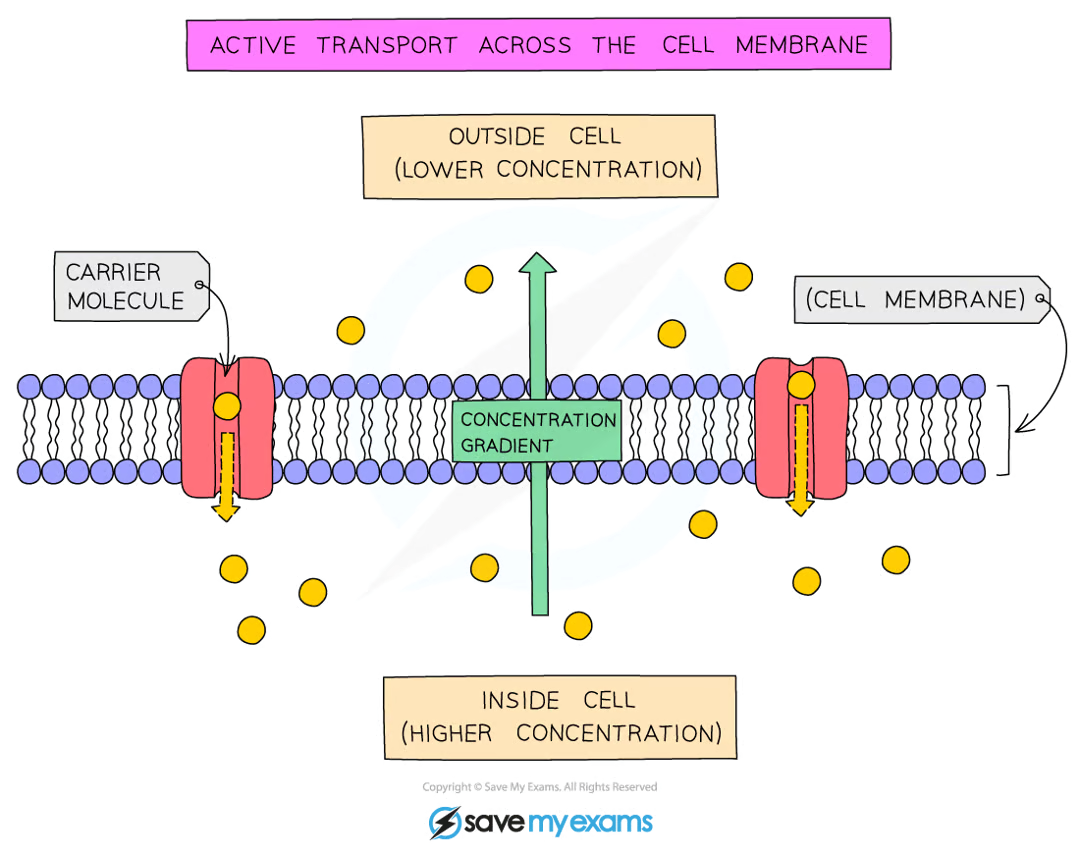
define active transport
the movement of particles across a cell membrane from a region of lower concentration to a region of higher concentration
active transport requires energy because particles are being moved against the concentration gradient
how does active transport work? what are some examples?
it involves protein carrier molecules that are embedded in the cell membrane
absorption of nutrients into the bloodstream from the small intestine / absorption of mineral ions from the soil into the root hair cells of plants
what factors affect diffusion?
surface area : volume ratio
great sa:v ratio → higher rate of diffusion
the bigger a cell or structure is, the smaller its sa:v ratio
cells which are adapted for diffusion have increased sa:v ratio in some way - e.g. root hair cells in plants, highly folded surface of small intestine
diffusion distance
the shorter the distance molecules have to travel, the faster the rate of diffusion
why blood capillaries and alveoli have walls which are only one cells thick
temperature
the higher the temperature, the faster the rate of diffusion
the molecules move faster as they have more thermal and therefore more kinetic energy
results in more collisions against the cell membrane
concentration gradient
the steeper the concentration gradient, the faster the movement across it will occur
this is because on the side with the higher concentration, more random collisions against the membrane will occur
PRACTICAL: Investigating diffusion & osmosis
METHOD
cut 2 equally-sized cubes of beetroot
same dimensions so they have the same SA:V ratio
rinse beetroot
put 5cm³ of water into 2 test tubes labelled A and B
keep test tube A at room temperature and transfer test tube B to a hot water bath at 90℃
leave them for 2 minutes then add a cube to each
after 10 minutes, observe the colour of the liquid
RESULTS
at the higher temperature, more of the pigment has leaked out
this is because:
the cell membrane of the beetroot cells has become damaged so more pigment can leak out
at higher temperatures, particles have more kinetic energy which results in faster movement of particles
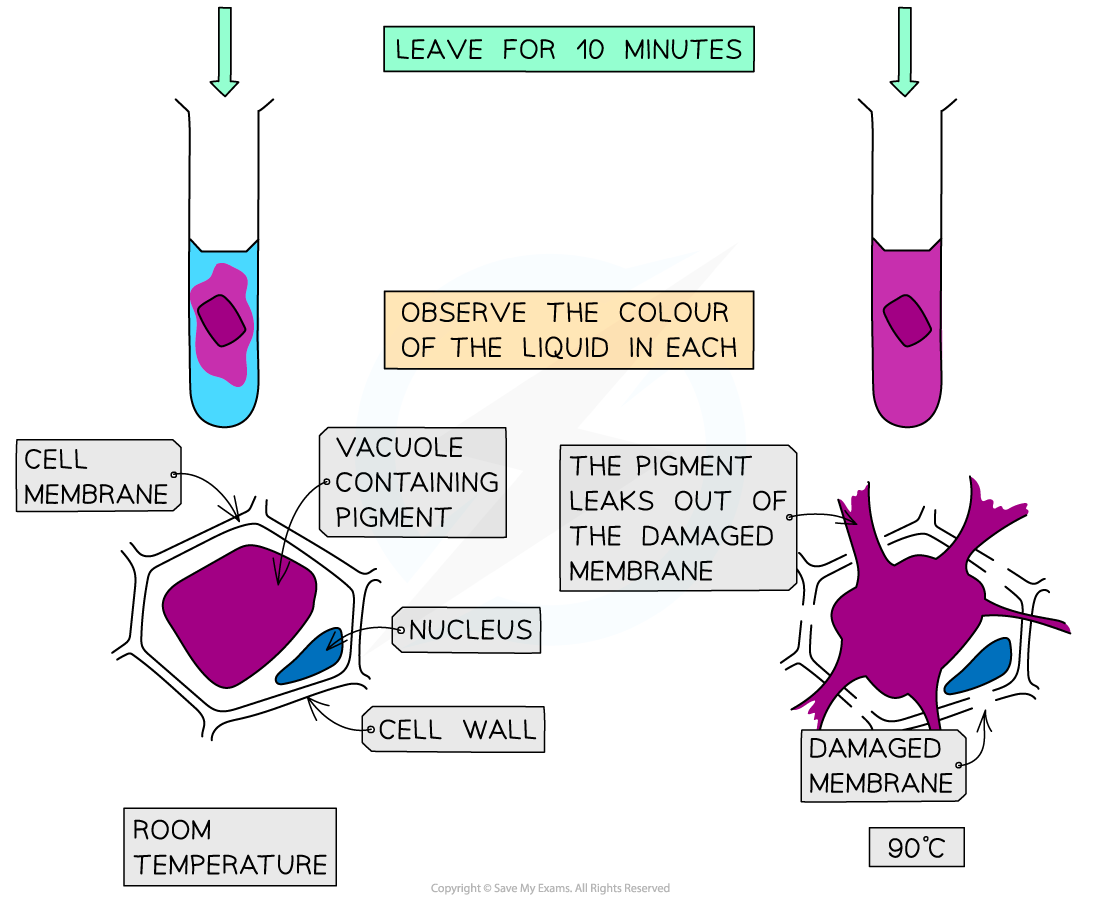
PRACTICAL: Investigating diffusion & osmosis
(CORMMS)
C - change the temperature of the water
O - the beetroot cubes will all be taken from the same beetroot
R - repeat the investigation several times to ensure results are reliable
M - observe the colour change of the liquid
M - after 10 minutes
S - control the volume of water used, the dimensions of the beetroot cubes and each cube will be blotted
PRACTICAL: Factors that influence osmosis
METHOD
prepare a range of sucrose solutions ranging from 0 mol/dm³ to 1 mol/dm³
set up 6 labelled test tubes with 10cm³ of each of the sucrose solutions
cut 6 equally-size cylinders of potato using a cork borer and knife
blot each one with a towel and weigh on balance
put 1 piece into each concentration of solution
after 4 hours, remove them, blot with paper towels and reweigh
RESULTS
calculate the % change in mass for each potato
the potato in distilled water will gain the most mass due to a steep concentration gradient, causing water to move into the cells by osmosis, increasing turgor pressure and making the potato firm
the potato in the strongest sucrose solution will lose the most mass as water moves out of the cells by osmosis, making them flaccid and the potato soft
PRACTICAL: Factors that influence osmosis
(CORMMS)
C - change the concentration of the sucrose solution
O - the potato cylinders will all be taken from the same potato
R - repeat the investigation several times to ensure results are reliable
M - measure the % change in mass of the potato cylinders
M - after 4 hours
S - control the volume of sucrose solution used, dimensions of potato cylinders, each cylinder must be blotted before it is weighed
define photosynthesis
the process by which plants manufacture carbohydrates from raw materials using energy from light
what is the equation for photosynthesis?

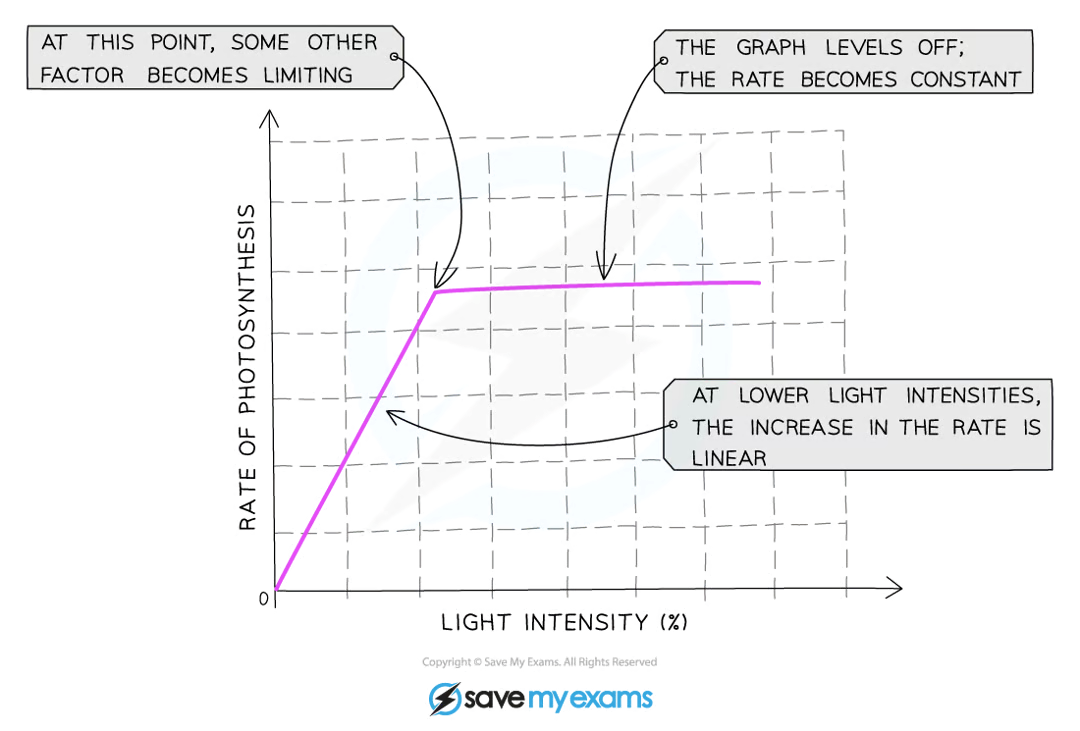
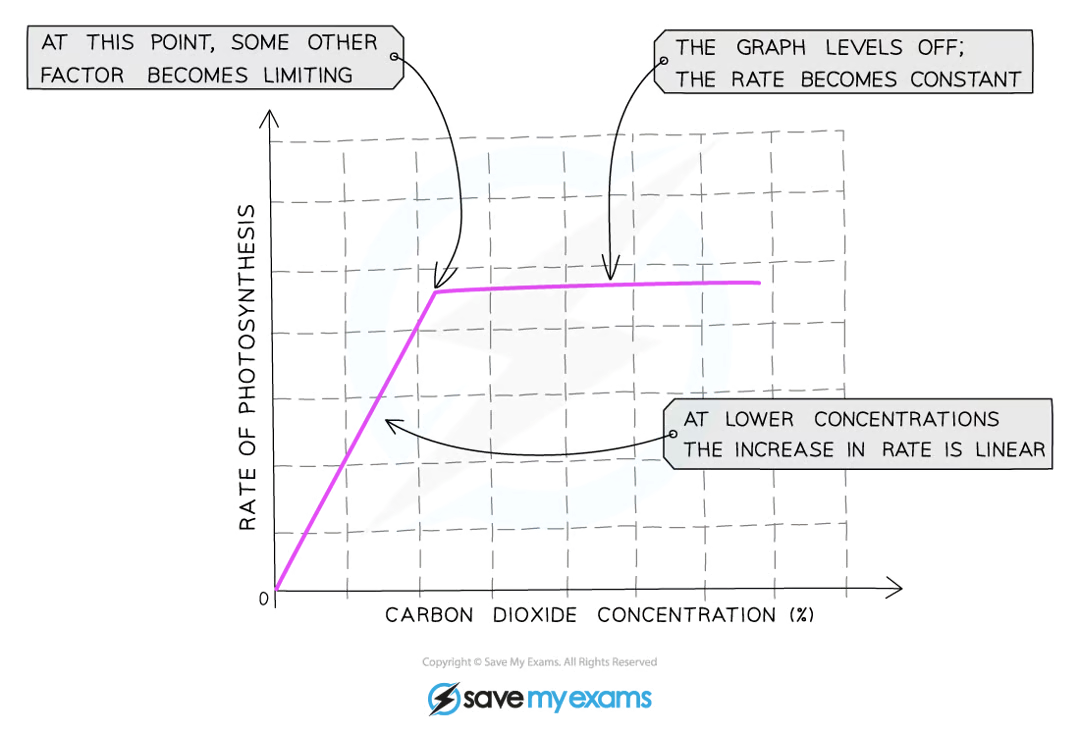
what factors limit the rate of photosynthesis?
temperature
light intensity
co2 concentration
how does temperature affect photosynthesis?
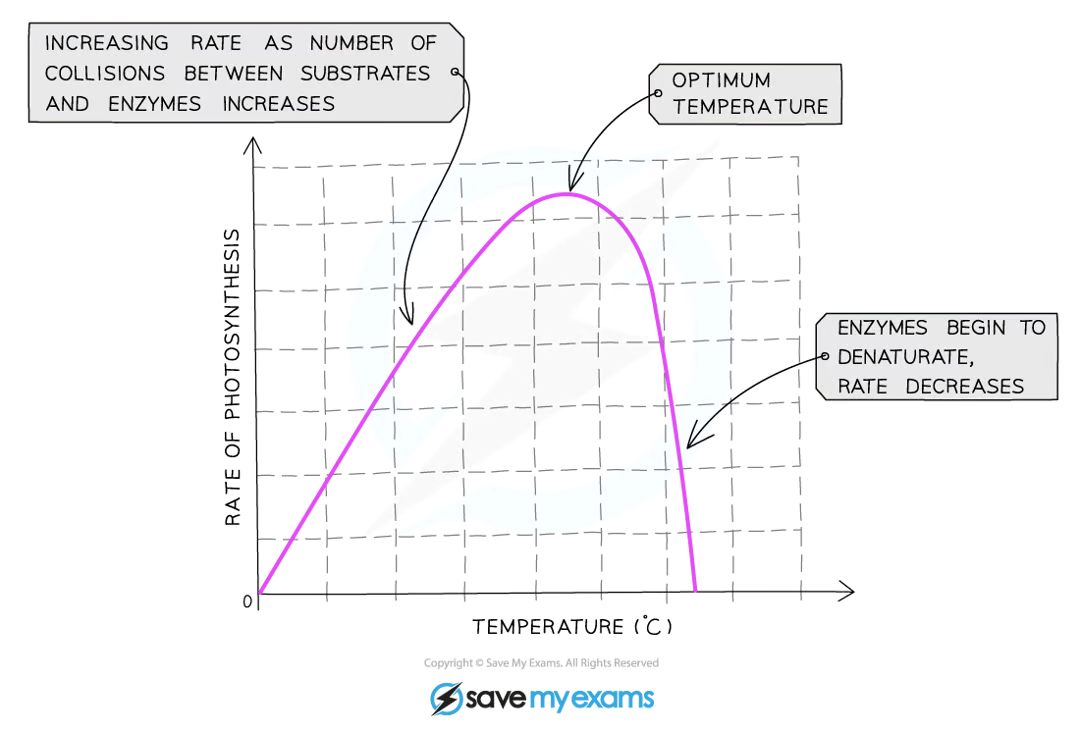
how does light intensity affect photosynthesis?
how does co2 concentration affect photosynthesis?
what are the leaf structures?
Structure | Description |
|---|---|
Wax cuticle | Protective layer on top of the leaf, prevents water from evaporating |
Upper epidermis | Thin and transparent to allow light to enter palisade mesophyll layer underneath it |
Palisade mesophyll | Column-shaped cells tightly packed with chloroplasts to absorb more light, maximising photosynthesis |
Spongy mesophyll | Contains internal air spaces that increase the surface area to volume ratio for the diffusion of gases (mainly carbon dioxide) |
Lower epidermis | Contains guard cells and stomata |
Guard cell | Absorbs and loses water to open and close the stomata to allow carbon dioxide to diffuse in, oxygen to diffuse out |
Stomata | Where gas exchange takes place: opens during the day, closes during the night. Evaporation of water also takes place from here. In most plants, found in much greater concentration on the underside of the leaf to reduce water loss |
Vascular bundle | Contains xylem and phloem to transport substances to and from the leaf |
Xylem | Transports water into the leaf for mesophyll cells to use in photosynthesis and for transpiration from stomata |
Phloem | Transports sucrose and amino acids around the plant |
what mineral ions are needed in plants?
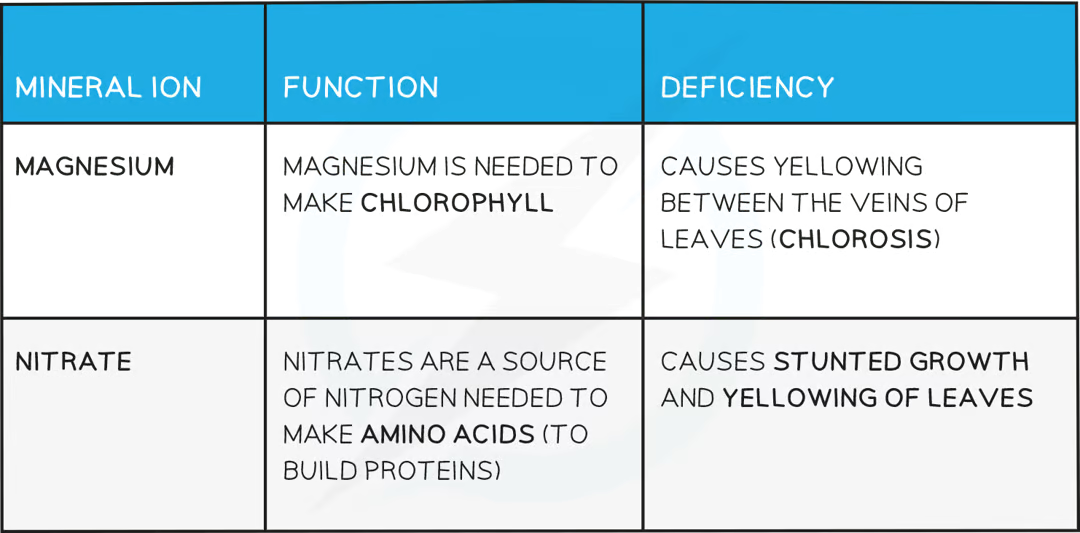
PRACTICAL: Evolution of Oxygen
METHOD
Take a bundle of shoots from a type of pondweed
Submerge them in a beaker of water underneath an upturned funnel
Fix a boiling tube with water and place it over the end of the funnel
As oxygen is produced, the bubbled os gas will collect in the boiling tube and displace the water
RESULTS
Show that the gas is oxygen with a glowing splint
The volume of oxygen/quantity of bubbles can be measured in order to investigate the rate of photosynthesis
PRACTICAL: Investigating Light and Photosynthesis
METHOD: PART 1
De-starch the plant by placing it in a dark cupboard for 24 hours
Ensures any starch already in present in the leaves will be used up
Partially cover a leaf of the plant with aluminium foil and place the plant in sunlight for 1 day
Remove the covered leaf and test for starch using the method below
METHOD: PART 2
Drop the leaf in boiling water
This kills the tissue and breaks down the cell walls
Transfer the leaf into hot ethanol in a boiling tube for 5-10 minutes
This removes the chlorophyll so colour changes from iodine can be seen more clearly
Rinse the leaf in cold water to soften the tissue
Spread the leaf out on a white tile and cover it with iodine solution
what are the 7 food groups?
carbohydrates
proteins
lipids
fibre
vitamins
minerals
water
what are the causes and effects of malnutrition?
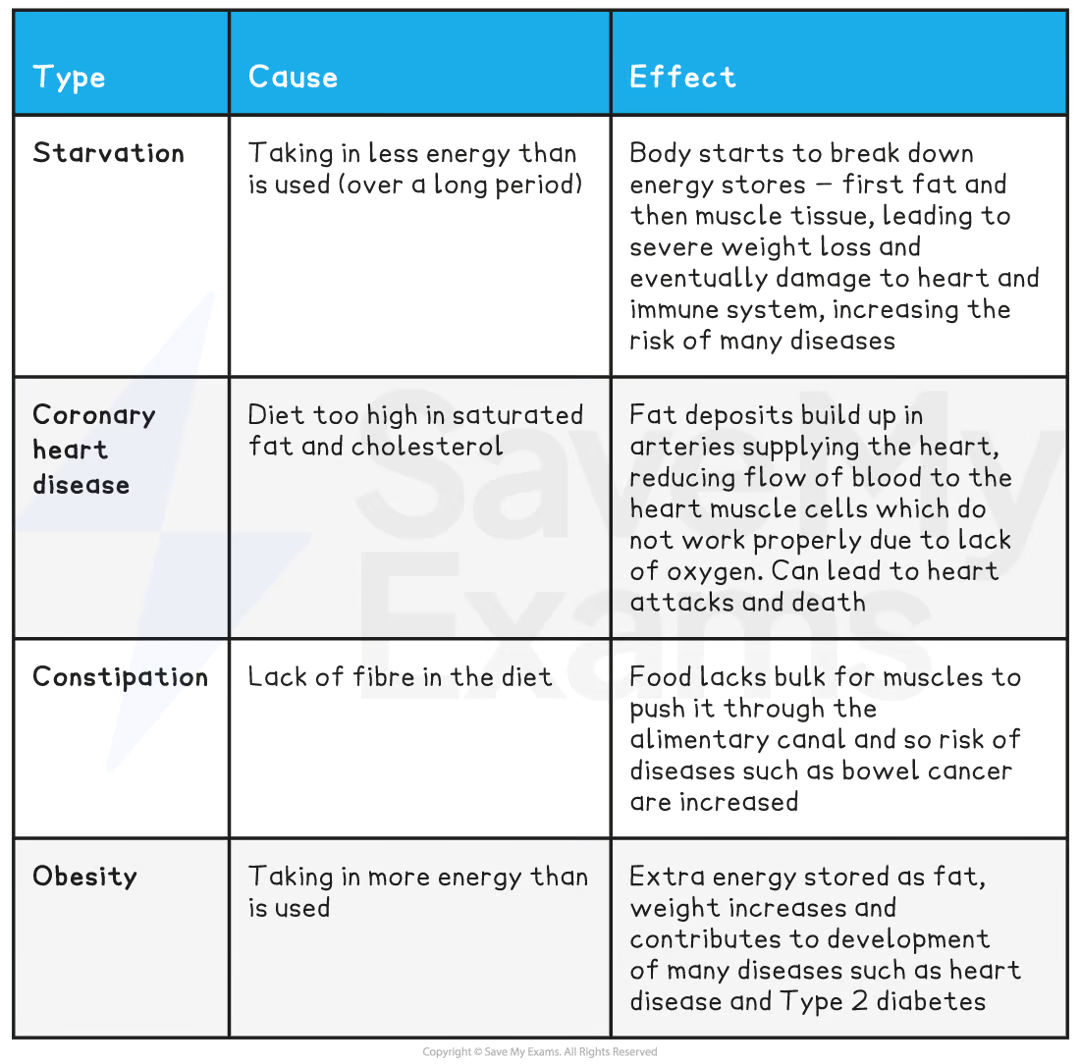
what is the function of carbs?
source of energy
what is the function of protein?
growth and repair
what is the function of lipids?
insulation and energy storage
what is the function of fibre?
provides bulk (roughage) for the intestine to push food through it
what is the function of vitamins and minerals?
needed in small quantities to maintain health
what is the function of water?
needed for chemical reactions to take place in the body
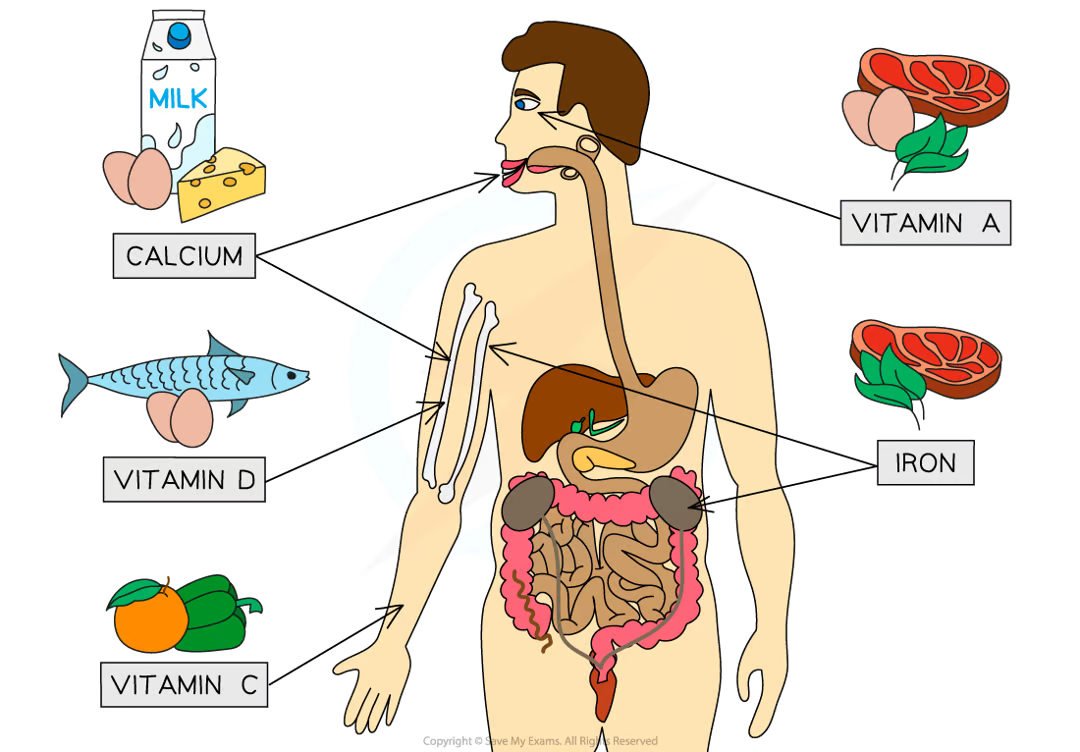
what are some examples of vitamins and minerals?
calcium - strong teeth and bones
vitamin D - helps the body absorb calcium
vitamin C - forms an essential part of collagen
vitamin A - needed to make the pigment in the retina for vision
iron - needed to make haemoglobin, the pigment in red blood cells that helps to carry oxygen
who needs more energy than the average person?
young people
more active
pregnant women
breastfeeding mothers
men
define digestion
a process in which relatively large, insoluble molecules in food are broken down into smaller, soluble molecules that can be absorbed into the bloodstream and delivered to cells in the body
label the digestive system
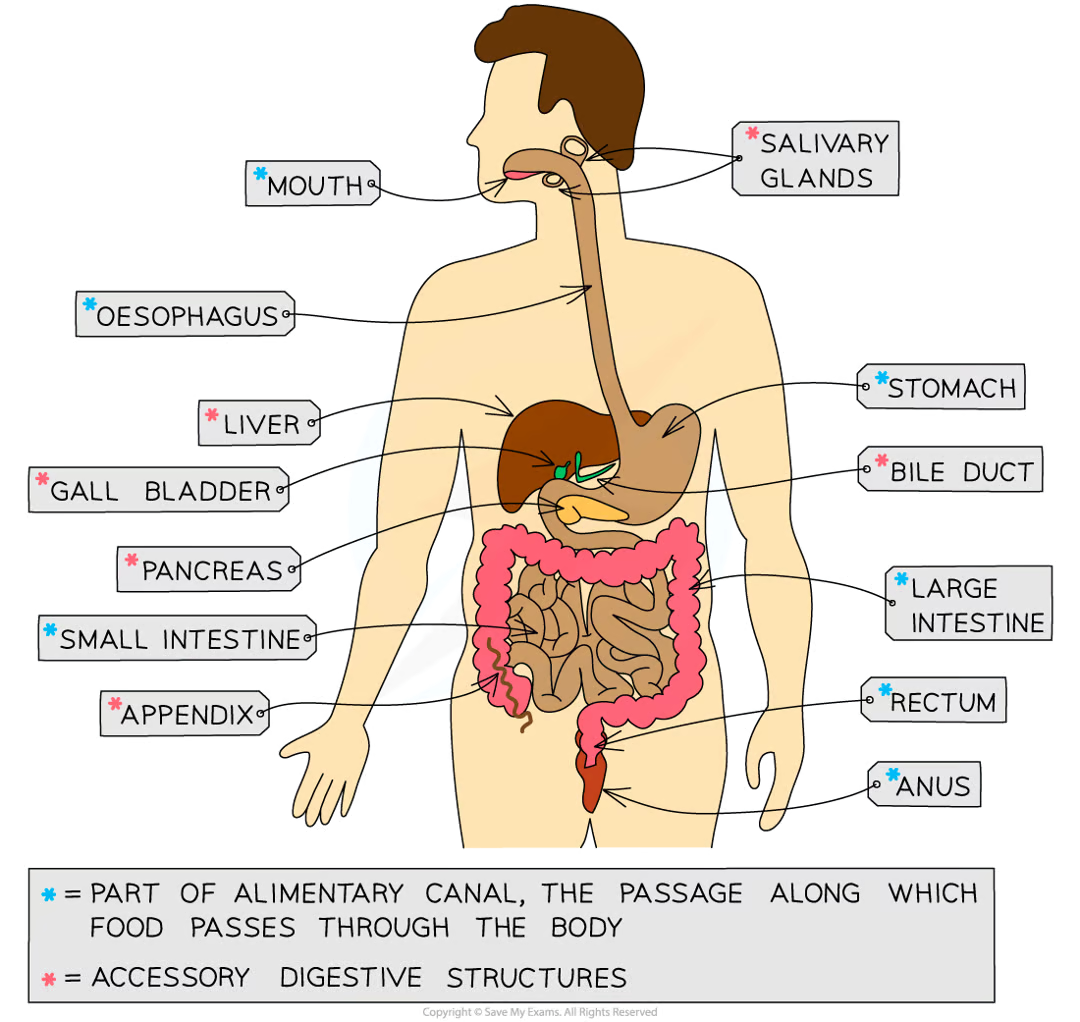
what are the functions of the organs that make up the digestive system?
Alimentary canal and accessory structures table
Structure | Function |
|---|---|
Mouth / salivary glands | Mechanical digestion: teeth chew food to break it into smaller pieces and increase its surface area to volume ratio Chemical digestion: amylase enzymes in saliva start digesting starch into maltose The food is shaped into a bolus (ball) and lubricated by saliva so it can be swallowed easily |
Oesophagus | The tube that connects the mouth to the stomach Wave-like contractions take place to push the food bolus down without relying on gravity |
Stomach | Food is mechanically digested by churning actions while protease enzymes start to chemically digest proteins. Hydrochloric acid is present to kill bacteria in food and provide the optimum pH for protease enzymes to work. |
Small intestine | The first section is called the duodenum; this is where digestion of the food exiting the stomach is completed by enzymes that are present in the duodenum lining and secreted by the pancreas The pH of the small intestine is slightly alkaline; around pH 8-9. The second section is called the ileum and is where the absorption of water and digested food molecules takes place; the ileum is long and lined with villi to increase the surface area over which absorption can take place |
Large intestine | Water is absorbed from the remaining material in the colon to produce faeces Faeces are stored in the rectum and exit the body via the anus |
Pancreas | Produces all three types of digestive enzymes: amylase, protease and lipase Secretes enzymes in an alkaline fluid into the duodenum for digestion; this raises the pH of fluid coming out of the stomach |
Liver | Amino acids that are not used to make proteins are broken down here (deamination), producing urea Produces bile to emulsify fats (break large droplets into smaller droplets), an example of mechanical digestion |
Gall bladder | Stores bile to release into the duodenum |
what are the six different stages of food breakdown?
ingestion - the taking in of substances into the body through the mouth
mechanical digestion - the breakdown of food into smaller pieces without chemical change to the food molecules
chemical digestion - the breakdown of large, insoluble molecules into small, soluble molecules
absorption - the movement of small food molecules and ions through the wall of the intestine into the blood
assimilation - the movement of digested food molecules into the cells of the body where they are used, becoming part of the cells
egestion - the passing out of food that has not been digested or absorbed (as faeces) through the anus
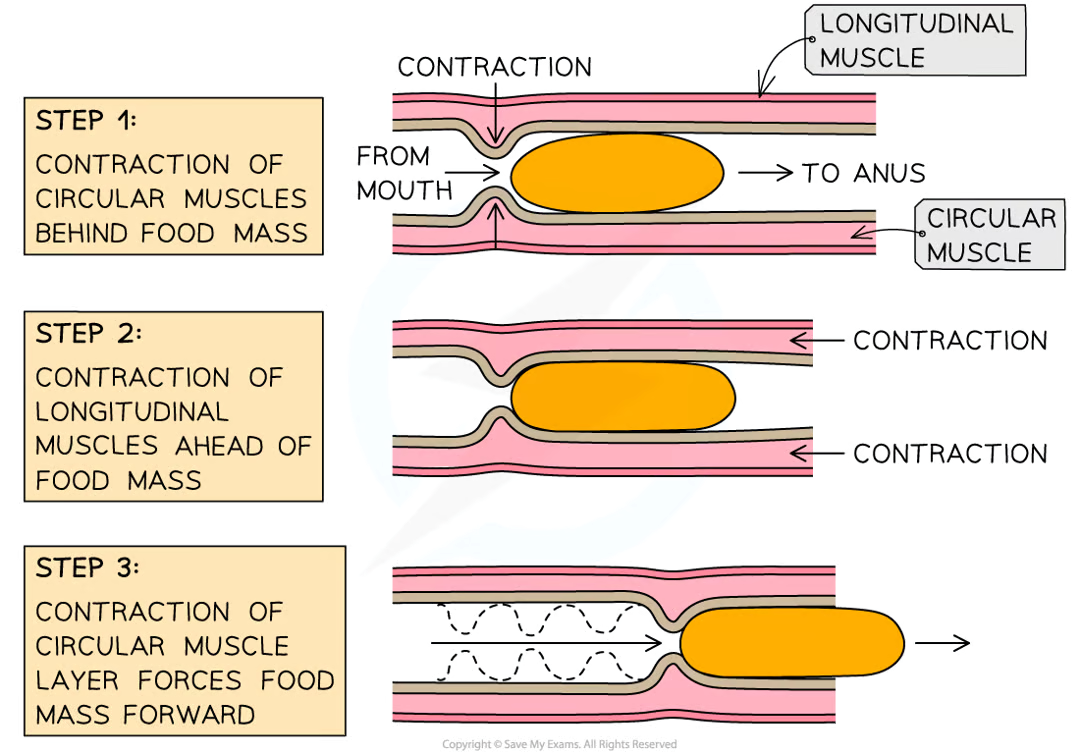
how does peristalsis work?
Firstly, muscles in the walls of the oesophagus create waves of contractions which force the bolus along
Once the bolus has reached the stomach, it is churned into a less solid form, called chyme, which continues on to the small intestine
Peristalsis is controlled by circular and longitudinal muscles
Circular muscles contract to reduce the diameter of the lumen of the oesophagus or small intestine
Longitudinal muscles contract to reduce the length of that section the oesophagus or the small intestine
Mucus is produced to continually lubricate the food mass and reduce friction
Dietary fibre provides the roughage required for the muscles to push against during peristalsis
what are the three main types of digestive enzymes?
carbohydrases
proteases
lipases
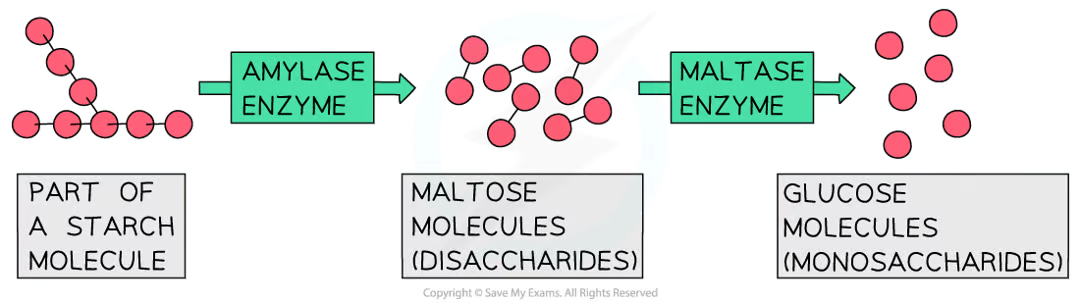
what do carbohydrases do?
enzymes that break down carbohydrates into simple sugars such as glucose
amylase breaks down starch into maltose
it is made in the salivary glands, the pancreas and the small intestine
amlyase from the salivary glands gets denatured in the stomach acid and must be replaced by amylase from the pancreas in the small intestine
maltase then breaks down maltose into glucose
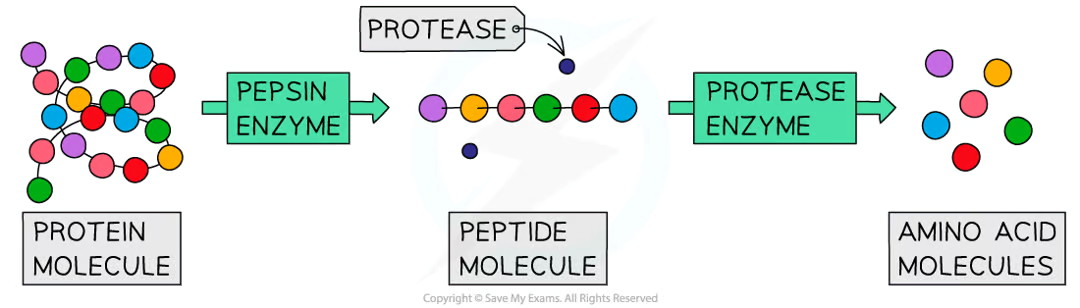
what do proteases do?
they break down proteins into amino acids
pepsin made in the stomach breaks down proteins into smaller polypeptide chains
protease enzymes made in the pancreas and small intestine break the chains into amino acids
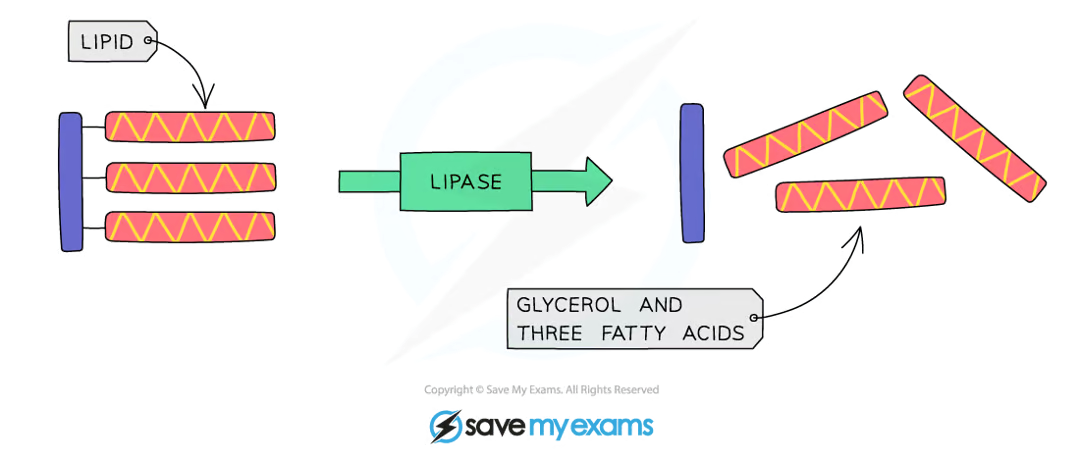
what do lipases do?
break down lipids to glycerol and fatty acids
produced in the pancreas and secreted into the small intestine
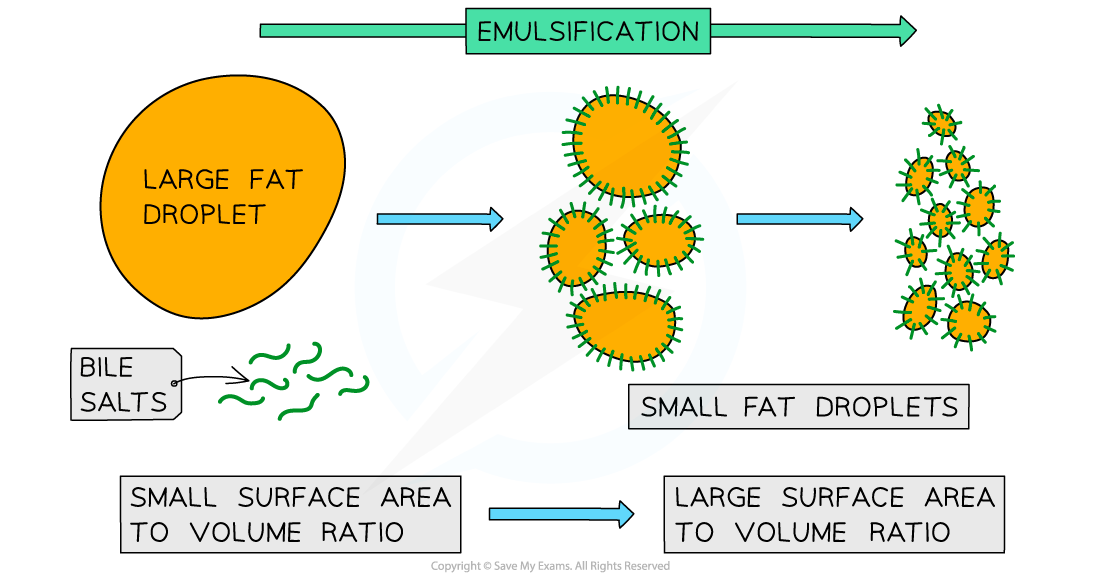
what is bile?
bile is an alkaline substance produced in the liver
before being released into the small intestine, bile is stored in the gallbladder
what are the two main roles of bile?
Neutralising the hydrochloric acid from the stomach
The alkaline properties of bile allow for this to occur
This neutralisation is essential as enzymes in the small intestine have a higher (more alkaline) optimum pH than those in the stomach
Breaking apart large drops of lipids (fats) into smaller ones (and so increasing their surface area)
This is known as emulsification
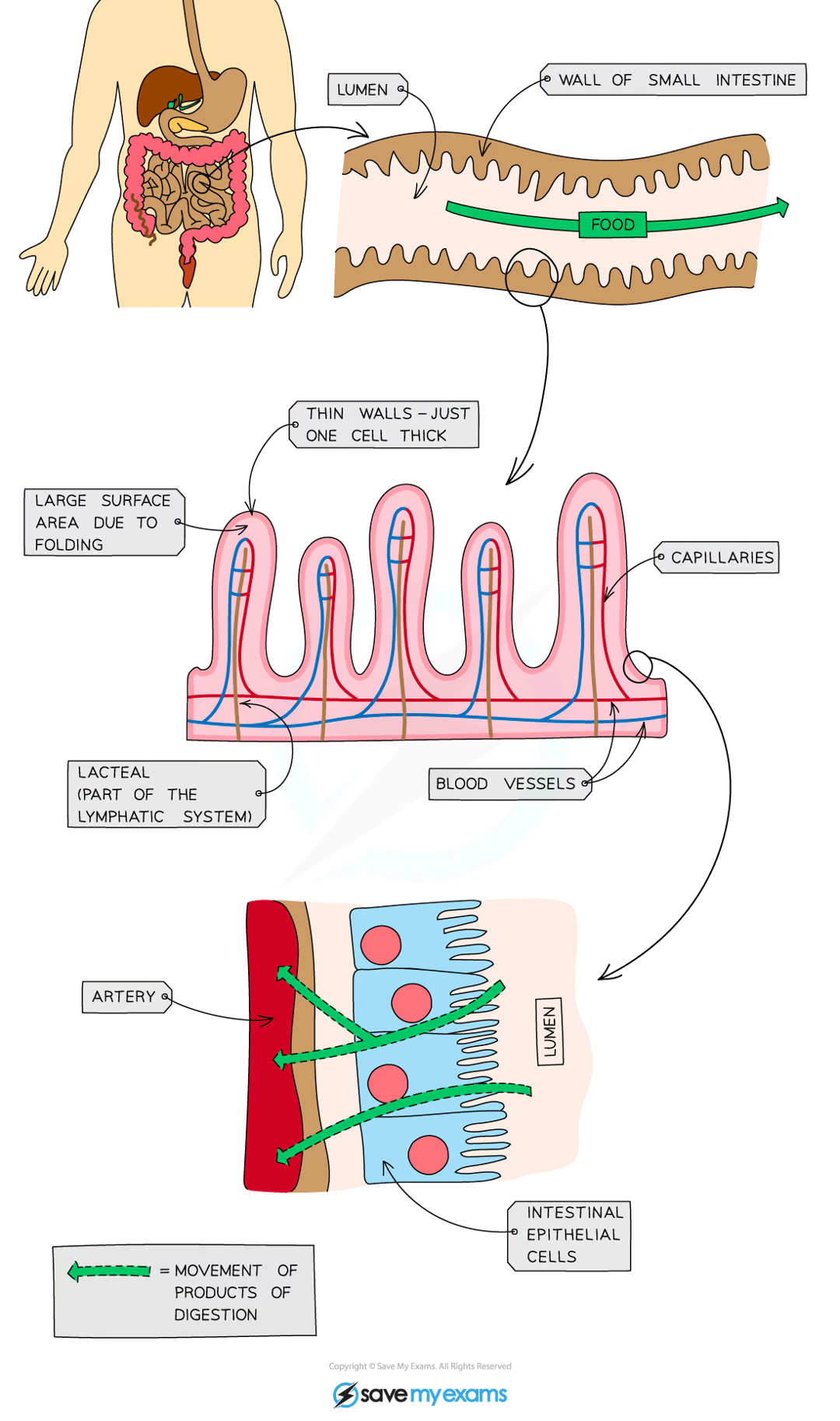
how is the small intestine adapted for absorption?
very long
has a highly folded surface with millions of villi
these adaptations massively increase the surface area of the small intestine, allowing absorption to take palce faster and more efficiently
how are the villi of the small intestine adapted for the rapid absorption of substances?
large surface area
microvilli on the surface of the villus further increase the surface available for absorption
short diffusion distance
the wall of a villus is only one cell thick
steep concentration gradient
the villi are well supplied with a network of blood capillaries that transport glucose and amino acids
a lacteal (lymph vessel) runs through the centre of the villus to transport fatty acids and glycerol
enzymes assist with chemical digestion
the movement of villi moves food along and mixes it with the enzymes
PRACTICAL: Energy Content of a Food Sample
METHOD
use a measuring cylinder to measure out 25cm³ of water and pour it into the boiling tube
record the starting temperature of the water using a thermometer
record the mass of the food sample
set fire to the sample of food using a bunsen burner and hold the sample under the boiling tube until it has completely burned
record the final temperature of the water
repeat the process with different food samples
RESULTS
the larger the increase in water temperature, the more energy is stored in the sample - use the equation