Topic 6 - Tissue Processing and Embedding
1/55
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
56 Terms
What is tissue processing
Any steps taken to prepare tissue for microtomy.
4 stages of tissue processing
Fixation, Dehydration, Clearing, Infiltration
What is dehydration
Stage where dehydrating agents remove all unbound water and fixative from tissue. Unbound water is the intracellular fluid and interstitial fluid
Major functions of clearing
Removes dehydrating agent and acts as solvent for the paraffin wax used for infiltration.
Also raises the refractive index of the tissue closer to that of glass, allowing for high power magnifiication
What is infiltration
Tissue impregnated with a support medium, usually paraffin wax, allowing for cutting of thin sections. After this stage, the tissue is ready for embedding
Dehydrating solution characteristics
Hydrophillic solutions that draw water out of tissues. The solutions are exchanged for increasingly concentrated solutions to dilute reminaing water
Most commonly used dehydrating agents
Ethanol, Isopropyl alcohol, Methanol
Dehydrating properties of methanol
Routinely used. Infinitely soluble with water, fast-acting, and relatively non-toxic. May cause hardening and shrinkage of tissue specimens.
Dehydrating properties of isopropyl alcohol
Miscible in water, but immiscible in salt solutions, so tissue must be washed following fixation. Isopropyl alcohol can be mixed with molten paraffin, so clearing agents are not always used/required. Relatively non-toxic, and does not harden or shrink tissue.
Methanol dehydrating properties
Similar to ethanol, but more hazardous (toxic).
Process of dehydration
Tissue subject to 50-70% alcohol immediately after fixation, followed by increasingly concentrated solutions (80, 90, absolute alcohol)
Waht makes a good clearing agent
Soluble in both dehydrants and paraffin
Role of clearing agent
Once tissue is dehydrated, clearing agent dissolves remaining alcohol, leaving tissue receptive to molten wax (non-polar)
Clearing agent refractive index
High refractive index (1.4-1.51), allowing the tissue to appear transparent when mounted on glass
Common clearing agents used in the histo lab
Xylene, Toluene, Chloroform, Xylene Substitute
Clearing agent properties of xylene
Routinely used. Fast-acting, and miscible with most solvents as well as paraffin. Over-exposure will harden tissue. Flammable and moderately toxic.
Clearing agent properties of toluene
Similar in action to xylene, but unlikely to harden tissues. More volatile than xylene (resulting in more vapours).
Clearing agent properties of chloroform
Sometimes used for C.N.S specimens that may become brittle when cleared with xylene (especially brain and eyes). Non-flammable, but highly toxic. Chlooform in the presence of oxygen may form phosgene, which is exceptionally hazardous (a dangerously poisonous gas).
Xylene substitute
Short-chain aliphatic hydrocarbons (the same class of chemicals as butane, petroleum jelly, and paraffin wax). They are less toxic and work almost as well as xylene. They are intolerant of water, so the final alcohol must be completely anhydrous.
What are universal solvents + examples
Solvents that are capable of dehydrating + clearing tissue
Most common are Tetrahydrofuran (THF) and dioxane
Role of infiltrating media
Forms a matrix and prevents distortion of tissue durig microtomy. Most common is paraffin wax
Common additives to paraffin wax
Plasticizers, Beeswax, Rubber, Resins
Role of plasticizers in paraffin wax
Used to make wax harder + facilitate ribboning
Role of beeswax in paraffin
Lowers melting point and makes sections sticky
Role of rubber in paraffin wax
Facilitates ribboning by increasing elasticity
Role of resins in paraffin wax
Makes wax harder/increasing melting point
Factors that effect tissue processing
Agitation, Heat, Vacuum/Pressure, Viscosity
How does agitation affect tissue processing
Ensures solutions surrounding tissues are not locally saturated, and helps avoid dead zones where solutions are not exchanged during processing.
How does heat affect processing
Speeds up processing, but must be careful as excess heat distorts microscopic details of delicate tissues. Recommended heat is the minimum amount to keep wax molten
How does vacuum/pressure affect processing
Pressure can force solutions into tissues, and vacuum can be used to “open” porous structures. The vacuum is particularly effective as an aid during infiltration; by lowering the pressure in the retort, the clearing agent is more easily replaced by hot wax, and the wax can more easily penetrate tissue structures.
Affect of viscosity on processing
Lower viscosity solvents work more quickly than wax
Pros + Cons of microwave processors
Pros: Improves turn around time, formallin + xylene free
Cons: Cost
Drawbacks of xylene-free substitutes
Do not clear alcohol as quickly as xylene, and is less tolerant of water contamination (ethanol must be 97% pure to be effectively cleared)
5 steps of processing with no clearing agent
Fixation
Ethanol dehydration (50% ethanol/water blend)
Continued dehydration/clearing (80% ethanol/20% isopropanol)
Isopropanol clearing (100% isopropanol)
High-temperature paraffin wax infiltration – the first wax bath should be held at 85 °C to effectively eliminate isopropanol (B.P = 82.5 °C at 760 mmHg)
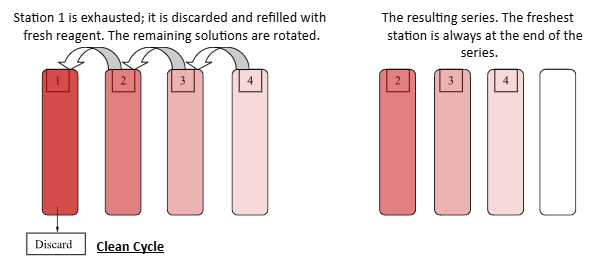
How are repeating reagents used to make sure they are the least contaminated
They are rotated to ensure the last station is the least contamination, and the first station in the series is discarded
How to remove paraffin from instrument lines
Flush lines with xylene to dissolve the wax and the flush lines with alcohol to dissolve the xylene
Most common cause of inadequate processing
Incomplete dehydration. Microtomy blocks have soft, mushy areas at the center of the tissue (where wax did not infiltrate). This creates a hole on the finished slide
What is the effect of a tissue that is over-processed
Tissue is brittle, and fragments easily. Microchatter is seen microscopically
What is embedding
Once tissue has been fully impregnated by paraffin, it is oriented in a mold where more wax is added and the block is left to cool in order to preserve the desired orientation. This provides a support medium for microtomy, facilitating the cutting of thin sections. It should also provide elasticity to resist the compression caused by microtomy.
Role of hard embedding wax
Higher melting point, and supports harder tissues. Can cut thinner sections
Role of soft embedding wax
Easier to ribbon, making them ideal for serial sectioning
Procedure of embedding
The tissue is removed from the cassette and examined to determine if any particular orientation is required.
A suitably sized mold is chosen (around 2-3mm bigger than the tissue on all sides to ensure good support), which is then partially filled with wax.
The specimen is placed centrally in the mold and transferred to a cold plate
. The tissue is gently held down in its desired orientation until a thin layer of wax at the bottom of the mold hardens, becoming opaque.
The cassette (with patient identifier) is placed on top of the mold, and additional wax is added. It is important that the wax fills all spaces in the tissue (otherwise the tissue will collapse during sectioning in those areas).
The block is now finished, and is placed on the cold plate until it is completely soli
Most critical step in embedding process
Orientation
Effects of improperly oriented tissue in embedding
Misdiagnosis (pathologist does not see area of interest, or the relationship between one tissue layer and another)
How to ensure specimen is embedded in correct orientation
Grossing person records instructions, or uses ink to indicate which side is placed downward
How should tissues with lumens be embedded
embedded to show a cross-section of the lumen.
How should tissues with layers be embedded
should be embedded “on-edge”, so that all layers are demonstrated on each slide
How to embed multiple pieces of tissue in the same cassette
placed side-by-side, and oriented with the epithelium facing the same direction. When embedding curettings, cluster them centrally, but do not overlap
How to improve microtomy when embedding an elongated tissue
offset the tissue slightly (up to a 30° angle from horizontal). This is especially important for tougher/harder tissues (bone, uterus) to prevent compression-type artifacts.
Paraffin block storage
Once a block has been cut and the slides are satisfactory, the block is sent for storage. Unlike the “wet” specimens (those stored in formalin that are discarded after 8 weeks), paraffin blocks are stored indefinitely.
How long should paraffin blocks be retained prior to discard
at least 30 years prior to discard. Blocks are routinely stored longer than that; it is dependent on the amount of storage space available.
Use of acrylic resins in histology
Section undecalcified bone, teeth, or to cut thin sections (less than 2µm) for light microscopy.
Examples of acrylic resins
Methyl methacrylate (MMA) or glycol methacrylate (GMA)
Can you use acrylic resins to embed for electron microscopy
No, they are unstable in eelctron beam
What resins should you use for embedding for electron microscopy
Epoxy resins. Also ideal for cutting semi-thin and ultra thin sections
Dangers of working with resins for embedding
use of potentially dangerous chemicals including organic peroxides (poisonous and potentially explosive). The setting/hardening of these resins involves a strongly exothermic reaction which can become hazardously hot. Always work in a fume hood to control the release of toxic vapours (carbon monoxide, nitrogen oxides, and ammonia are most notable).