chemistry-unit 3
1/88
Earn XP
Description and Tags
unit-3:structure of atom
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
89 Terms
the existance of atom has been proposed since time of early____and____philosophers
→indian and greek
the word atom is derived from the greek word_____meaning______
→’a-tomos’,meaning uncuttable or non-divisible
_____thought that all materials are made up four things called earth,air,water,fire in the 5th BC
→Empedocles
plato is the student of_____and the teacher of______
→socratus,aristotle
_____adapted empedocles theory and wined the term elements to describe the four substances
→plato
______introduced the idea that elements can be differentiated based on properies
→aristotle(also adapted empedocles concept of 4 atoms)
democritus was a student of____________
→leuccipus
_____proposed the atomic theory of matter,taught the theory of atomism(indivisible and indestructive atoms)
→democitus
the work of leucippus and democritus was further developed by_____
→epicurus(mad the idea more generally known)
what are the five major point of the atomic ideas of the greek philosophers
→all matter is composed of atoms which are bit of matter too small to be seen(democritus)
→there is a void w/c is an empty space b/ atoms(leucipuss)
→atoms are completely solid
→atoms are homogenous with no internal structure
→atoms are d/t in sizes,shapes and weight(democritus)
the generally accepted model of democritus is called__________
→the solid sphere
what is the drawback of early greek philosophers???
→tried to understand the nature of the world through reason and logic but not with experiment
____are statements based on repeated experiment or observation that describe or predicts a range of natural phenomena
→scientific laws
_____are those laws of nature relevant to chemistry
→chemical laws
the law of conservation of mass is also known as_________
→law of indestructibility of matter
____discovered that mass is neither created nor destroyed in a chemical reactions
→lavoisier’s
_____proposed the law of definite proportion
→joseph louis
the law of definite proportion is also known as_____________-
→proust’s law or law of constant composition
what does the law of conservation of mass states??
→states that in a given that is closed to transfer matter,the amount of matter stays unchanged
→the mass of the element states the mass of any element at the beginning of a reaction will equal mass of that element at the end of reaction
what are the two laws stated on the conservation of mass??
→the law of definite proportion
→the law of multiple proportion
what does law of definite proportion states??
→the molecular weight of a substance is the sum of the atomic mass of all atoms in molecules
→states that every chemical compound contains fixed and constant proportions of constituent elements
what does the law of multiple proportions state??
→states that when 2 cpds are formed from the same elements w/c react with fixed mass of the other,are in the ratio of small whole number
____is the way how we interpret facts about atoms
→atomic theory
____proposed his atomic theory in 1803,based on certain observation and experimental results.
→john dalton(English man)
dalton studies the weight of various elements and compounds,he perceived that matter is always combined in a________based on______________
→fixed ratios,based on weight(volume incase of gases)
what provided further support for proust’s law of definite proportion
→that chemical cps always contain the same proportion of elements regardless of amount(dalton)
____also proposed that there could be more than one combination of two elements.
→dalton
what are the new theories that are proposed by dalton??
→elements are made up small particles called atoms
→atoms can neither be created nor destroyed
→all atoms of the same elements are identical and have the same mass and size(Isobars)
→atoms of d/t elements have d/t masses and size(Istopes)
→atoms combine in small whole numbers to form cpds
_____postulated that each element has a characteristics type of atom that differ in properties from each atoms of all other elements
→dalton
the presence of protons was predicted by________in his anode experiment in 1886.
→Eugene goldstein
the discovery of electron and charge to mass ratio was discovered by____in 1847.
→thomas
the charge to mass ratio(precisely)was discovered by_____in 1909
→robert millikan
the presence of nucleus was discovered by____in 1920
→Ernst rutherford
the discovery of newtons by_____________in 1932
→james chadwick
mention the draw back of dalton’s atomic theory
→the indivisibility of an atom was proved wrong(proton,electron and neutron)
→atoms of the same elements are similar in all aspects(by dalton),atoms of same elements vary in their masses and densities(isotopes)
→atoms of d/t element differ in mass..also proved wrong(isobars)
→atoms of d/t elements combine in simple whole number ratios to form acpd,this wasn’t observed in complex organic cpds like sugar and protein molecules
mention the major modern atomic theory postulates
→elements are made of small particles called atoms
→atoms can’t be destroyed nor created during ordinary chemical rxn
→all atoms of the same elements have the same atomic number but may vary in mass number due to presence of istoopes
→atoms of d/t elements are d/t
→atom combine in small whole numbers to form compound
the presence of positively charged particles in an atom has been predicted by eugan goldstein(1886)based on___________________
→electrical neutrality of an atom
anode rays are a beam of__________moving from a node toward the cathode in a specifically designed discharge tube at_______
→positively charged,low pressure
how are anode rays produced??
→they are produced when cathode rays(high speed electrons)collide with neutral gas atom or molecule in a discharge tube,knocking out electrons and leaving behind positive ions.
what is the direction of travel of anode rays??
→they travel form anode towards the cathode opposite to the direction of cathode rays(pass through canals(holes)in perforated cathode
what is the charge of anode rays??
→positive charge,the charge to mass ratio was much smaller than that of electron,and it’s NOT CONSTANT depends on the nature of residual gas in tube
what is the behaviour of anode rays in electric and magnetic field??
→they are deflected by both electric of magnetic fields but init he opposite of the cathode rays due to their positive charge, their deflection is much smaller than that of cathode rays b/c of their significant larger mass
______is an early experimental electric discharge tube with potential vaccum
→Crooke’s tube
crooke’s tube was invented by_________in which around 1869-1875_____was discoverd
→william crookes,cathode rays(stream of electrons)
the two metal tube in the discharge tube are called_____
→electrodes
the electrode connected to the positive terminal is called____
→anode
the electrode connected to the negative terminal is called____
→cathode
the electrodes were connceted by_____
→low pressure,because at high pressure there was no electric flow in the air in the discharge tube
what does the high voltage provides for the atoms??
→provides energy for the atoms to split or breakup
when both electrodes are connected to high voltage…what happens??
→current start flowing
the rays that are emitted from the direction of cathode are called_________
→cathode rays
____performed several experiments in the discovery of electron
→JJ thomson
what is the first experiment that thomson has done(on cathode rays)??
→it’s is studying how cathode rays travel in the discharge tube by placing small object b/n the anode and cathode
what does the formation of shadow of the object on the side of cathode revealed??
→that the cathode rays travel in a straight line
why does the cathode ray travel towards the anode??
→because they are attracted by the positively charged anode
what was the second experiment of jj.thomson(on cathode rays)??
→it was performed by placing a light paddle wheel b/n cathode ans anode to study the particulate nature of the cathode ray
what did jj.thomson expect when placing a light paddle wheel b/n cathode and anode??
→he assumed that if the cathode rays could move the paddle wheel,then they have a particle nature(and actually observed that they have a particle nature of the cathode rays w/c includes they have kinetic energy and mass)
what was jj.thomson’s 3rd exp’t for??
→to investigate the charge of the cathode rays by passing the cathode ray through electric and magnetic fields
in electromagnetic wave,the electric and magnetic fields are___________
→are perpendicular to one another
a moving electric charge generate_______where as magnetic field induces_________mov’t,producing______
→magnetic field, electric charge,electric current
what proved that the cathode rays are negatively charged???
→upon passing through an electric field the cathode rays bent towards the positive plate
what happens when the magnetic field is applied perpendicular to the path of cathode rays??
→they get deflected towards the northpole(+) of the magnet
by measuring the extent of deflection of cathode rays in electric or magnetic field of various strength,jj thomson was able to calculate was able to calculate the mass to charge ratio of the particle to be________
→1.76×108c/g
mention properties of cathode rays
→travel in a straight line from cathode to anode
→cast shadow of metallic object in their path from
→cause mechanical motion on light puddle wheel in their path,which indicates they possess kinetic energy and have material particle
→charge to mass ratio of the ray is constant
→upon passing through the electric and magnetic field the cathode rays bend towards the positive plate showing that they’re negatively charged
→have penetrating power,when cathode rays are allowed to pass through thin metal foil a glow is seen behind the metal foil indicating that they have good penetrating power
following the discovery of electron, jj thomson proposed a new model called________in 1004
→plum pudding model
mention the key postulates of the plum pudding model
→positive sphere(atom is a sphere of a uniform positive charge)
→embeded electrons(the negatively charged electrons are embeded with in positive sphere)
→stable arrangement(the electrons are held in place by electrostatic force and can vibrate around their fixed position)
→overall neutrality(the charges balance exactly making the atom electrically neutral as they go closer to the outer portion of the atom)
electrons are held place by electrostatic force and vibrate around their fixed position w/c thomson used to explain_____
→light emission
the positive charge in a region was greater than_________and the electron would pull more towards the center region of the atom
→neighbouring negative charge
mention the validty of thomson’s atomic model
→thomson’s atomic model could successfully explain electrical neutrality
→now it’s known that positively charged nucleus exist in the nucleus
_______carried out a series of experiment using electrically charged oil droplets
→robert millikan(1909)
in robert’s experiment some fine oil droplets were allowed to be sprayed into the chamber by_____
→atomizer
the air in the chamber is subjected to ionization by______,the electron produced by_______,air attack themselves to the oil drops,when a sufficient amount if electric field is applied.w/c can just balance the_____force acting on oil, the drop remains suspended in the air
→x-ray,ionization,gravitational
milikan observed that the smallest charge on cathode rays was approximately_____and the charge on each drop was an integral multiple of that value.
→1.59×10-19c
robert milikan concluded that using smallest possible charge________and thomson’s charge to mass ratio_____,he calculated the mass of electrons
→1.59×10-19c,1.76×108c/g
what is the mass of electrons(as robert calculated)
→9×10-28g
the mass of an atom is equal to_____________concentrated inside the nucleus
→the mass of protons
_____was the first scientist to predict the reason for discrepancy of the mass of d/t atoms,he predict that along with protons,there were some other neutral particles present inside the nucleus.
→rutherford
in a single famous experiment,ernst rutherford showed____
→explicity
thomson’s model of an atom was correct
false
mention some features of discovery of the nucleus
→by 1920,with discovery of electron and proton it was thought that inner structure of atom was complete(nope not complete)
→d/t atoms have d/t number of protons,hence d/t atomic masses
x-particles consists of______and_______tightly bound together
→two protons and two neutrons
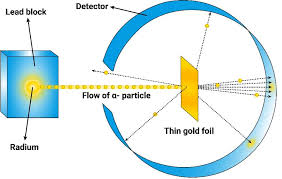
in 1920,_____targeted a stream of positively charged x-particles average thin gold foil and inspected how x-particles were scattered by the foil
→rutherford
rutherford’s results werenot consistent with_________________-
→dalton’s atomic theory(stated that the mass and positive charge are distributed uniformly throughout the volume of atom)
what does rutherford’s experiment result showed??
→strongly suggested that both mass and positive charges are connected in a tiny minute fraction of the volume of an atom(opposite to dalton’s atomic model)
coming to the stability of an atom,rutherford explained that revolving electron is under influence of two types of forces,,,w/c is:-
a)the electrostatic force of attraction b/n the nucleus and the electron
b)the centrifugal force directed away the revolving electron
mention the two feature of the types of forces(mentioned on flashcard 85)
→are equal and opposite and keep the electron in equilibrium in the path
mention the key postulates of rutherfords atomic model
-an atom has tiny,dense positively charged called nucleus
-nucleus contains nearly all atom’s mass but occupy a minuscule volume(the nucleus)
-the rest of atom is mostly empty space(empty space)
-electron revolve around the nucleus in a circular path(planetary motion)(electron orbits)
-the total charge of the nucleus is exactly balanced by the total negative charge of electron w/c makes the atom electrically neutral(charge neutrality)
rutherford’s model of atom is called______
→planetary model
rutherford experiment (diagram)
yea just look at thattttt