BIOL 2056 - Signal transduction and intracellular signalling
1/36
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
37 Terms
methods of signal transduction: membrane translocation
clustering of proteins at the cell membrane
enzymatic reactions dependent on conc of substrates
drives transformation of the cell
MECHANISMS CONTROLLING PROTEIN TRANSLOCATION
PROTEIN INTERACTION
receptor binds to ligand —> conf change
increased ability of protein to interact with receptor
LIPID INTERACTION
generates new surfaces of CSM which allows protein to bind to CSM
LIPID THETHER
fatty acid tail linked to a protein
help to tether protein to the membrane
often one lipid tail not enough to lipid tails modified to stabilise protein in memb.
lipid tethering
MYRISTOLATION
activation pathway for fatty acids
activate w CoA then remove met and couple myristific acid —> N terminal glycine residue can now attach to CSM
cotranslational modification
mediates cell apoptosis
highly controlled —> only active when caspase activated and allows Bid to attach to CSM —> bingd in other proteins —> cyt c release —> cell death
PRENYLATION
occurs in CAAX motifs on carboxyl terminus
C residue is the isoprenoid attachment site
A is any aliphatic AA
X is any AA
initiated by attachment of farnesyl or geranyl geranyl isoprenoid lipid to Cys residue farnesyl transferase or geranylgeranyl transferase
E.g: the prenylation of Ras results in the active form
Ras association w CSM
3 isoforms of Ras: H, N, R
H ras has 2 prenyl groups which forms a more stable association with the membrane than the R ras which only has 1 prenyl group and a polybasic region
Serine residue in the middle of the R ras’ polybasic region can be phosphorylated causing a negative charge—> repels from the CSM and falls off
when it falls off it translocates to teh other organelles and can induce apoptosis
phosphorylation regulated by PKc
methods of signal transduction: protein phosphorylation
requires an Oh group therefore occurs at serine, threonine and tyrosine
increases size and surface charge of molecule
protein kinase - writer, phosphatase - eraser
METHODS OF INCUDING CHANGE:
conformational change
can disrupt to promote molecular interaction
leads to shape change
protein readers
reader proteins may only interact with the protein when its in the phosphorylated form
E.g: PIN1 —> proline isomerase that switches proline from cis —> trans which causes downstream signalling
what can phosphorylation do?
conf shape change —> activation of kinases
induce new interactions
prevent protein binding
change subcellular localisation of proteins
activation of degradation pathway
sequestration
sequestration to membranes can be used to inhibit processes
E.g: hormones/notch signalling which requires the translocation to the nucleus
adrenergic stimulation
ADRENERGIC STIMULTION
activation of both a adreno receptors on smooth muscle cells and b adreno receptors on cardiac, liver, adipose and skeletal muscle
increased cardiac output
increased O2 supply
glucose to the blood
lipolysis for energy
increased muscle tension
decreased stomch/intestinal activity
decreased peripheral blood flow
adrenal release into blood from adrenal gland
nervous adrenaline at postganglionic synapse
adrenal gland
ADRENAL GLAND ADRENALINE
similar to nervous adrenaline
chromatin cells release epinephrine in an endocrine manner
adrenaline secreted from the medulla
OTHER HORMONES
cortex of the medulla secretes cortisol, andonesterone and androgens
cortisol: stress response, metabolism and immune regulation
Aldosterone (mineralocorticoid): Regulates sodium and potassium balance, and blood pressure
Adrenal androgens: Contribute to sex hormone balance.
adrenal pathway
adrenaline binding to the receptor activates the heterotrimeric G protein which is tethered to the membrane
activation of GPCR causes a conformational change so that the intracellular domain can interact with the a subunit of the G protein which binds GDP
GDP replaced with GTP which causes the dissociation of the a subunit with the bg subunit.
the a subunit and GTP bound stimulates the formation of cAMP by binding to adenylyl cyclase
cAMP acts as a 2nd messenger and binds to the regulatory subunits of pKA, revealing the catalytic subunits whcih leads to the phosphorylation of glycogen phosphorylase
pKA
4 subunits, 2 regulatory, 2 catalytic
allosteric activator
when cAMP increase becomes active
phosphorylates at serine and threonine residues
the pseudo substrate keeps the enzyme in the inactive conformation —> looks the same as the substrate except for a glycine residue
phosphorylation of phosphorylase causes a twist in the structure which causes activation —> phosphorylated at serine 14
OTHER EFFECTS OF PKA
switches off glycogen synthesis
pyruvate kinase inactivated and blocks glycolysis
inactivates an enzyme that dephosphorylates glucose so that it is released into the blood
switching off of PKA
beta adrenergic receptor kinase phosphorylates the GPCR N terminus tail
the receptor is internalised causing G alpha inactivation
a low pH vesicle causes the ligand to fall off teh receptor
GTP is hydrolysed
receptors are back in teh GDP bound state
causes reassociation of the beta gamma subunit
activation of phosphodiesterase
hydrolyses cAMP to make AMP
other effects of adrenaline
effects cardiac tissue
effects adipose tissue
effects smooth muscle tissue
alpha adrenoreceptors
BOTH
gunaine nucleotide exchange factor
3 subunits
A1 ADRENRECEPTORS
G alpha q type
found in
A2 ADRENORECEPTORS
G alpha i type receptor
decreases concentration of cAMP
blocks insulin release —> activation of PKA required for exocytosis of insulin
beta adrenoreceptors
G alpha s type
stimulates production of cAMP
will cause glucose to be released into the blood but not insulin —> this helps to maintain high blood sugar levels
beta gamma subunit
important in signalling too, not just the dissociation from the alpha subunit
regulates the potassium channel which makes depolarisation more difficult
adrenalines effect on smooth muscle
SMOOTH MUSCLE
binding of adrenaline to the a1 adrenoreceptor activates phospholipase C which hydrolyses PIP2 releasing IP3 and DAG
PIP2 is a lipid made up of an inositol head group
IP3 diffuses into the cytoplasm and triggers the release of calcium from the sarcoplasmic reticulum
the IP3 receptor is a tetramer bound to the ER
the N terminus binds IP3
when calcium dependent protein calmodulin binds to cam kinase it can induce muscle contraction
DAG stays in the membrane and recruits PKC to the membrane —> combined with the increase in intracellular calcium, DAG becomes active
PKC is a serine threonine kinase that phosphorylates various target proteins with the following effects:
smooth muscle contraction
glycogenolysis
inhibition of glucose synthesis so that glucose released in the blood is not just taken up again
modulation of ion channels
adrenalines effect on cardia cells
INCREASE IN HEART RATE
helps to get glucose and fatty acids to the muscles
same pathway as pka but also acts on calcium channels
ion channel allows Ca2+ into the cell
ca2+ stimulates the release of more Ca from the RYR receptor
pka also phosphorylates phospholambam which prevents the RYR receptor from activating SERCA which will uptake calcium
increased Ca in the cell allows for faster relaxation for the next contraction
on adipose tissues
RELEASE OF FA
in adipose
adrenaline acts on a2 and b3 receptors
same pathway activates adipose specific enzymes which metabolise lipid droplets
activation of pKa phosphorylates perilipin and a conformational shape change causes it to fall off
HSL is phosphorylated too and leads to translocation of lipid droplets as theyre broken down
fatty acids in blood can now be used for energy
EPAC
regulates cAMP levels in the cell as well as PKA
activation of PKA can control transcriptional output through KREB
receptor tyrosine kinase structure
insulin receptor is an example of a receptor tyrosine kinase
approx 50 diff types of RTKs which control a range of outputs
all single pass transmembrane proteins except the insulin receptor which is a dimer
extracellular binding site which varies and an intracellular catalytic domain which is similar across receptor type —> a protein kinase that specifically phosphorylates tyrosine
activation of receptor tyrosine kinases
ligand binding causes a conformational change
receptors dimerise (can take different modes depending on the receptor) and it causes each receptor to phosphorylate one another —> caused by catalytic domain flipping from the inactive to the active state
after cross phosphorylation it allows the receptors to phosphorylate the tails of the receptors on tyr residues
different modes of dimerisation
LIGAND DEPENDENT
no external receptor interaction
RECEPTOR DEPENDENT
not through ligand interaction
BI LIGAND INTERACTION
ligands bind with 2 interaction surfaces that bring the receptors together
ACCESSORY PROTEIN
ligand and receptor interaction which requires an accessory protein
SH2 domains
domains present in around 100 proteins
interact with specific phosphorylated tyrosine molecules
PTB binds phosphorylated tyrosine doains but structurally different from SH2
2 interaction sites:
pocket domain drives specificity
pTyr pocket binds to pTyr
although the pTyr pocket fits any pTyr it will not bind unless it fit into the specificity pocket
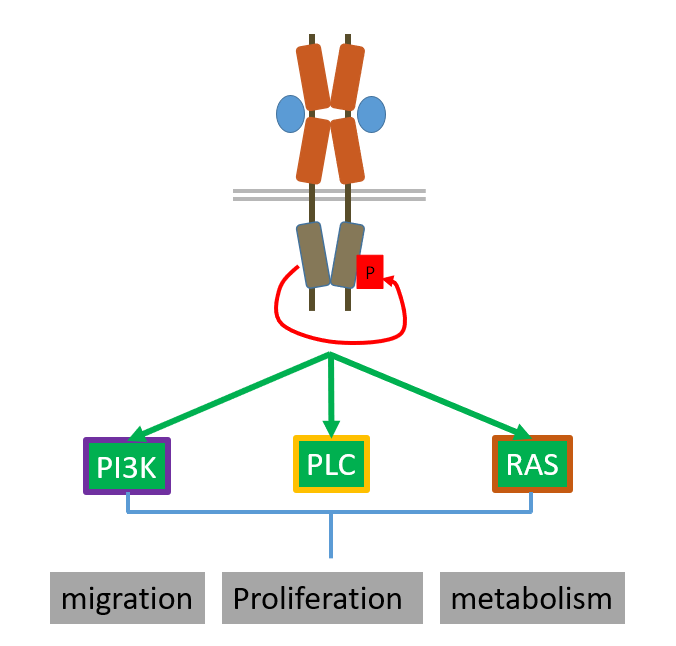
PDGF receptor acts in 3 pathways:
PI3K pathway
PLC gamma pathway (DAG and IP3)
MAPK pathway
adaptor proteins
some RTKs recruit an adaptor protein to the receptor induced by phosphotyrosine binding instead of phosphorylating their own tails
adaptors are then post translationally modified or phosphorylated to mediate signalling
switching off
caused by internalisation of the receptor
Casitas B lineage protein has an SH2 domain which recognises one of the phosphorylated tyrosine residues on the receptor
Cbl will ubiquitinate the receptor for degradation
the ubiquitinated receptor gets internalised to the endosomal system
either goes to the lysosome or gets recycled into the membrane
there are also phosphatases which can dephosphorylate the phosphorylated tyrosine kinase residues
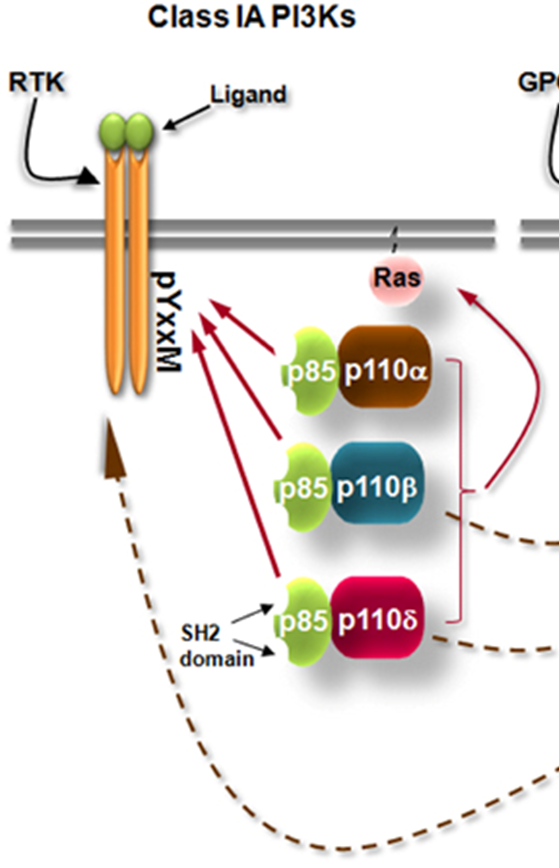
PI3K
PI3K is an enzyme which phosphorylates PI(4,5)P2 —> PI(3,4,5)P3 which cats as a second messenger
there’s a dimer of the regulatory p85 domain and a catalytic domain
the p85 domain contains the SH2 domain which binds RTKs
p85 domain also acts like a clamp for when ligand or receptor not bound, p85 domain will bind to the catalytic domain to suppress it
it also stops the enzyme from degradation and enables recruitment of the inactive enzyme to receptor through SH2
middle t (an antigen which phosphorylates lipids instead of proteins), GPCRs and receptor tyrosine kinases all activate PI3K
PIP3 production
phosphorylation of the YxxM motif on the receptor allows it to bind to the SH2 domain of the p85
this brings the enzyme to the membrane and brings it to its substrate
once the p110 (enzyme bound to p85) bought to the membrane it relieves the inhibition and allows it to produce PIP3
switching off of PIP3
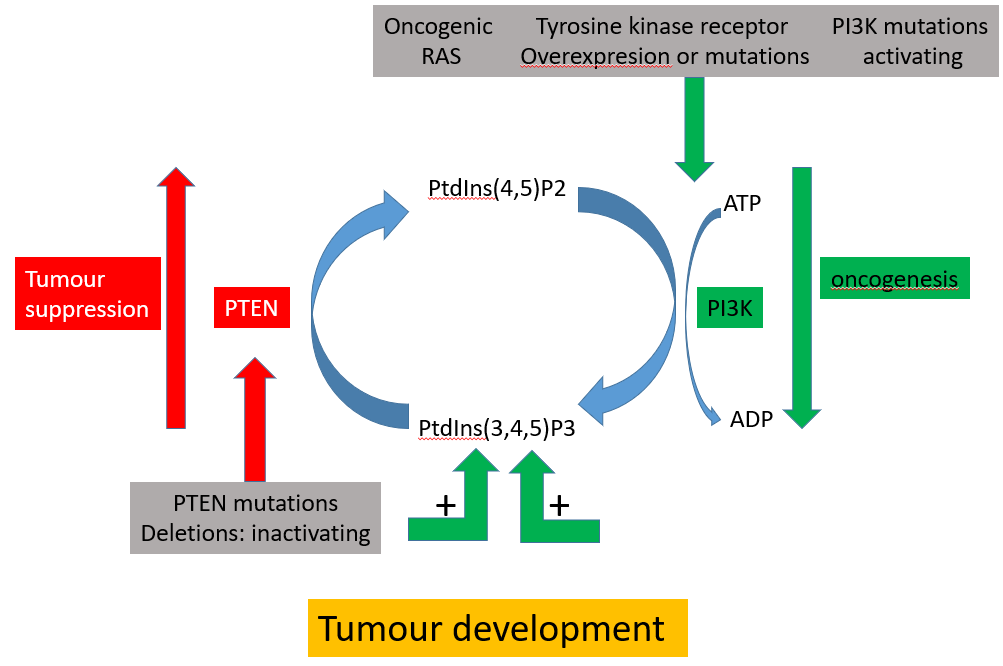
PTEN can remove the phosphate group at the 3 position
what does PIP3 do?
the pH domain binds to PIP3 and are 2 beta sheets which mediate the protein interactions
there is a famous pH domain that it binds to —> PKB which is a serine threonine kinase
PKB
has a specific pH domain that binds to PIP3 only and becomes phosphorylated at the t308 and s473
can now phosphorylate a number of serine/threonine
works antagonistically against adrenaline pathway as it activates phosphodiesterase which hydrolyses cAMP
can also activate GSK1 which increases glycogen synthesis as the PKB pathway increases the uptake of glycogen
it does this by putting glucose transporters into the membrane
this is because it drives other proliferative pathways which require glucose
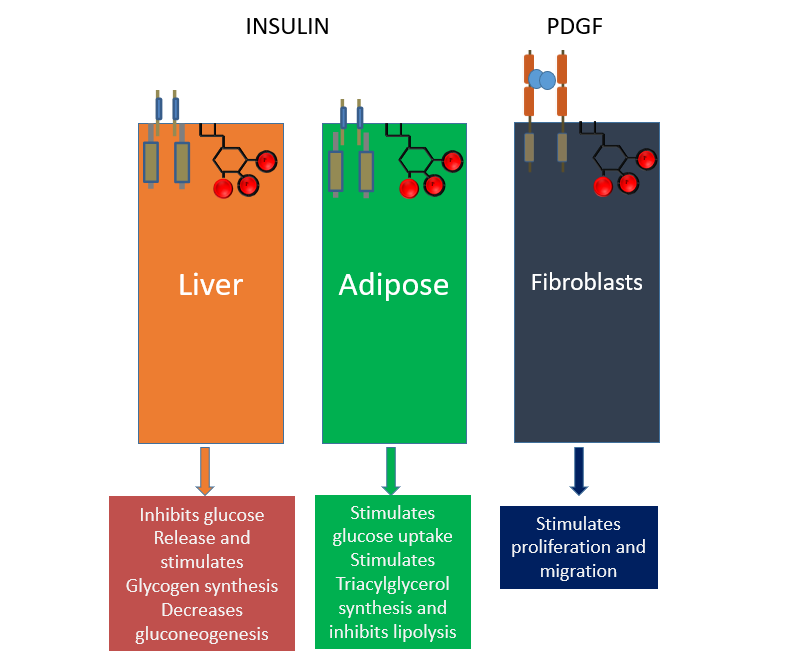
different tissues
receptor coupled PIP3 signalling can drive differential responses in different cell types
LIVER
it inhibits glucose release and stimulates glycogen synthesis, decreasing gluconeogenesis
ADIPOSE
it inhibits lipolysis
FIBROBLASTS
it drives proliferation
oncogenic PIP3 pathway
highly deregulated
PI3K mutated
the mutation/overexpression of tyrosine kinase drives more PI3K
PTEN is deactivated
PLC pathway
beta family activated by GPCR but gamma form activated by RTKs, as it has SH2 domains
phosphorylated SH2 domain of PLC gamma allows it to be recruited to the receptor
then it can hydrolyse PIP2 into DAG and IP3
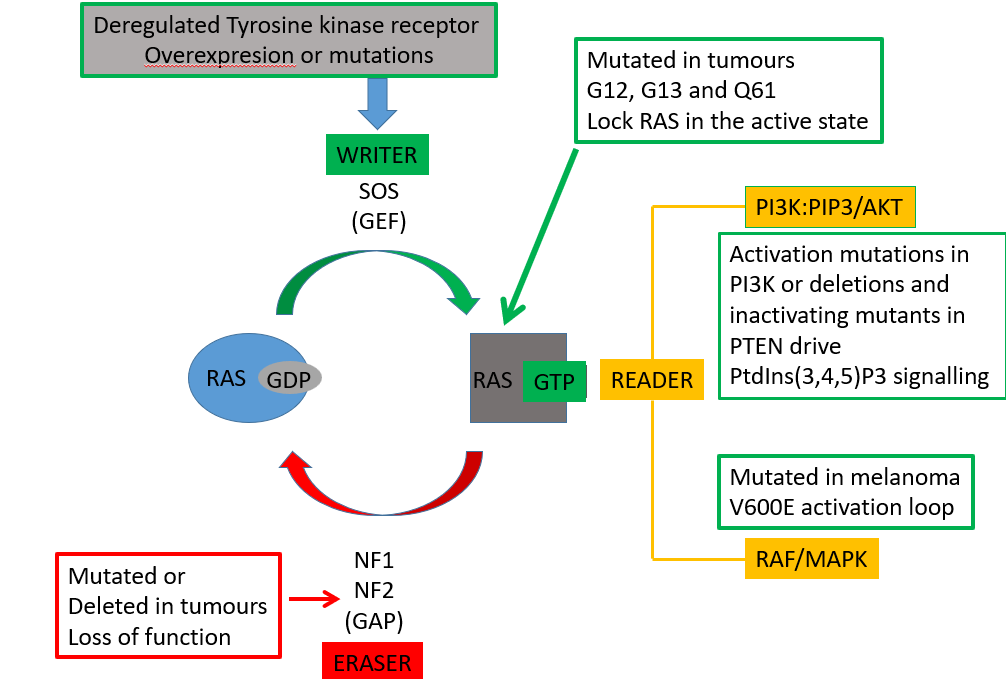
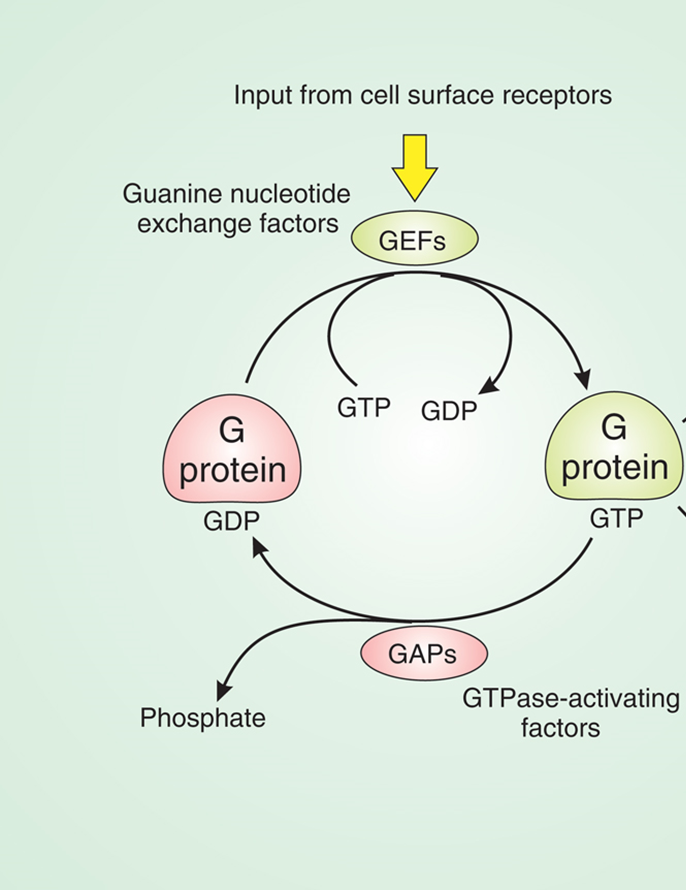
RAS pathway
small momomeric G protein
inactive in the GDP bound state, active in the GTP bound state which allows it to interact with downstream proteins
the Ras pathway controls proliferation
CONTROL OF PROLIFERATION
GRB2 is an adaptor protein with SH2 and SH3 domains
the SH2 domain will bind specific phosphorylated tyrosine residues on the RTK
GRB2 recruits SOS via SH3 domain interaction
SOS is activated at the membrane and acts as a guanine nucleotide exchange factor so RAS has GTP not GDP
when theres a conformational change in switch 1 and switch 2 domains RAS can bind many proteins including PI3K, RAF/MAPK
active RAS
can bind a kinase called RAF kinase
leads to a cascade which eventually leads to activation of MAPK
MAPK can phosphorylate transcription factors and cause cells to proliferate
active RAS can also bind to PIP3K to phosphorylate PIP2—> PIP3
there are 3 isoforms of RAS in different tissue types:
HRAS in brain and muscle
NRAS in the gut the lung and the thymus
RRAS in the testis the thymus
RAS is often mutated in tumors, most frequently at G12, G13, Q61 which stops RAS from hydrolysing GTP
locks the RAS in the active state driving PI3K and MAPK pathways
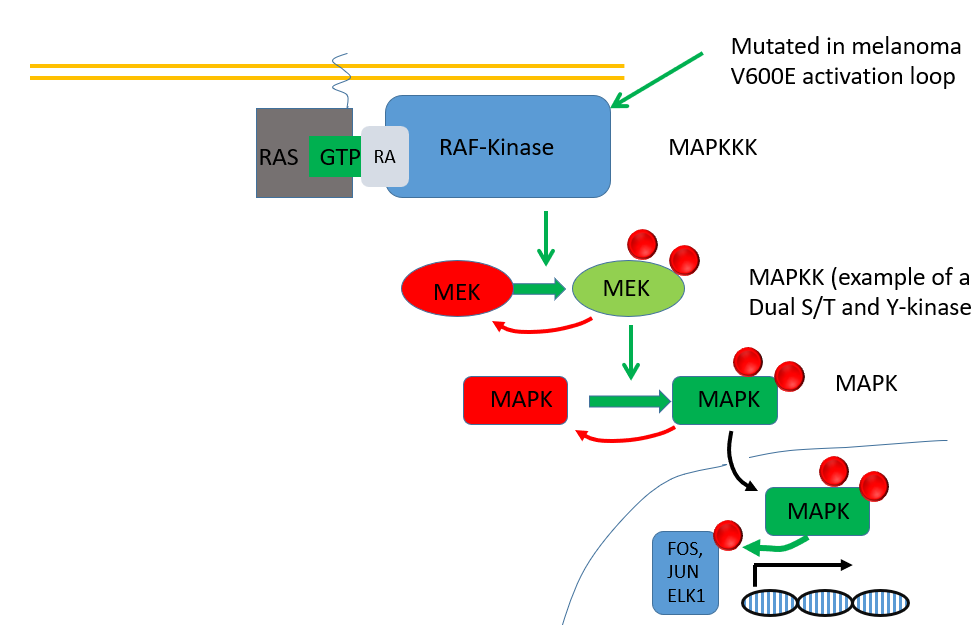
switching off RAS
RAS has its own GTPase activity but this is very slow so it relies on a GAP (eraser)
however, these GAPs are mutated in tumors
a mutation in the arginine finger will aid at pos G12, G13, Q61 will prevent the Arg finger from interacting with it and activating GTPase activity (via steric hindrance)