Sed Pet Week 14
1/25
Earn XP
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
26 Terms
Outsized pores
Large, irregular pores surrounded by smaller ones in a rock, often seen in carbonates. Might be preserved by calcite lining
Fenestral porosity
Happen in muddy carbonates, outsized pores are filled with cement. These pore spaces are kept open by gas bubbles escaping upward (from organic decay) that get trapped and allow for cementation
Vadose zone
unsaturated (air filled) environment located between the surface soil and the saturated zone
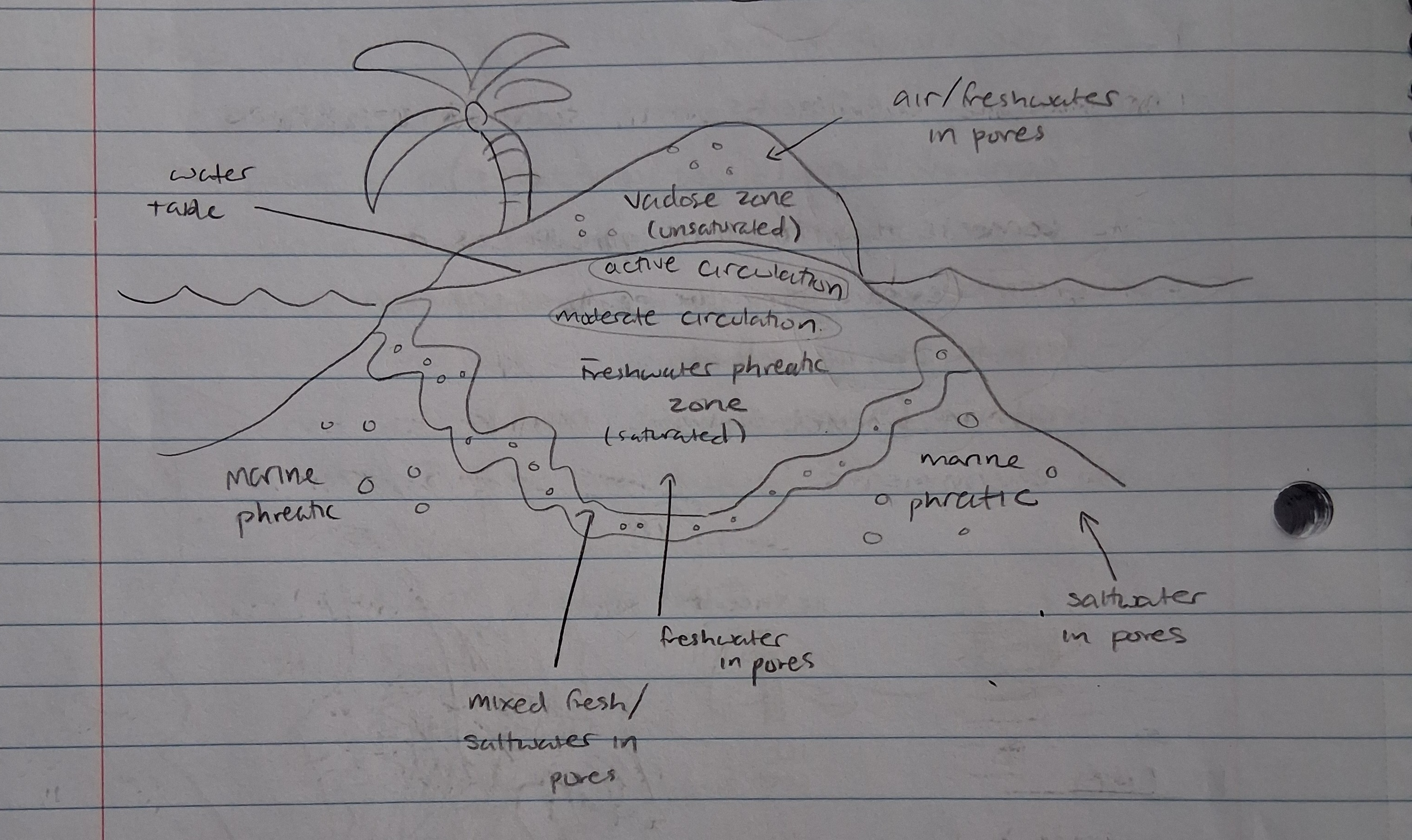
Phreatic zone
Saturated zone below the soil. Often the source of aquifers

Carbonate ion concentration
If D > 1: the water is supersaturated (susceptible to carbonate formation
If D < 1: the water is undersaturated (not susceptible to carbonate formation)
If D = 1: water is exactly saturated
Aragonite dissolves faster than calcite. True?
True. This is why you see more carbonate ions in seawater in aragonitic environments; it is more soluble
in some cases, aragonite will revert to calcite
Carbonate dissolves more readily under
Cold, acidic conditions as opposed to warm ones (solubility increases with pressure and depth)
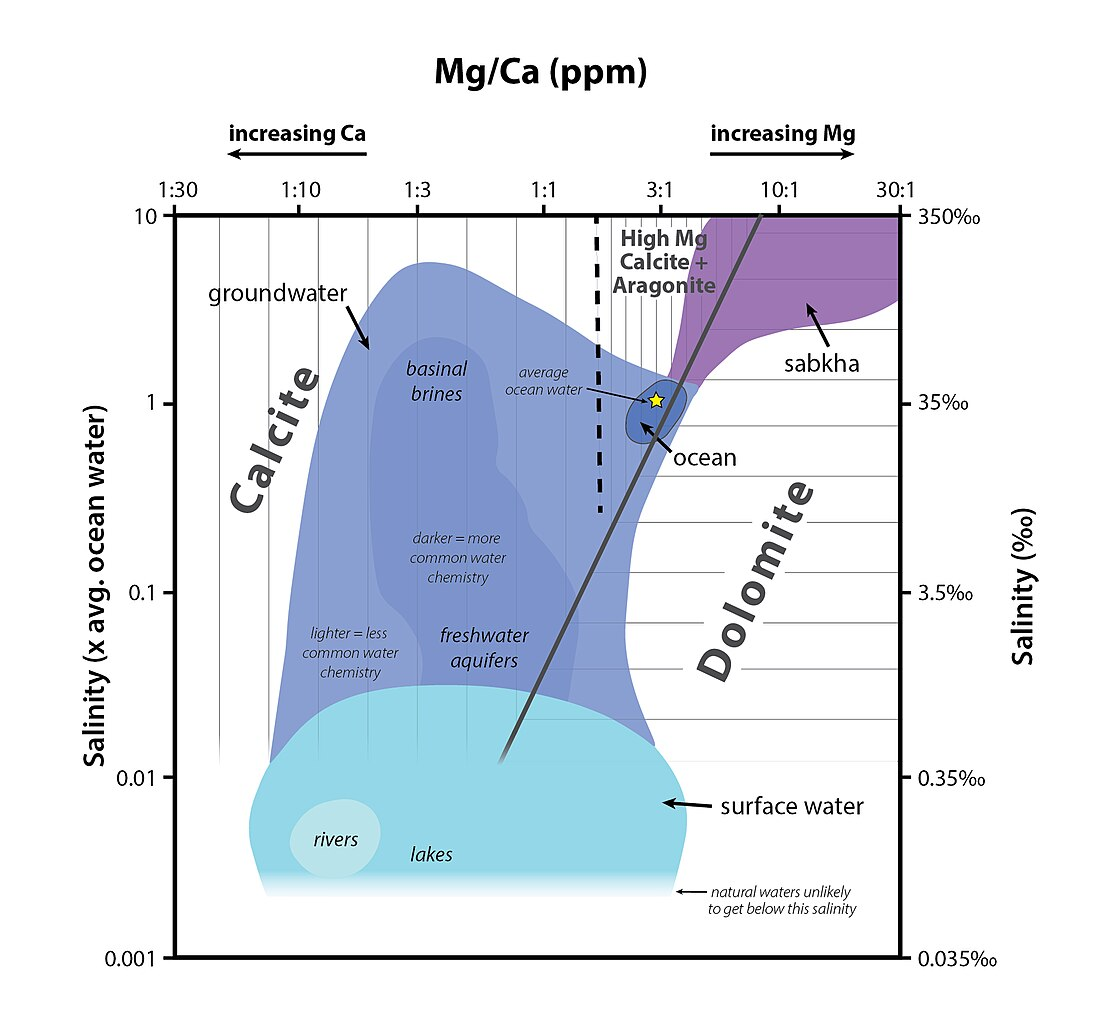
Normal marine salinity
3.5%; 35 grams of salt per liter
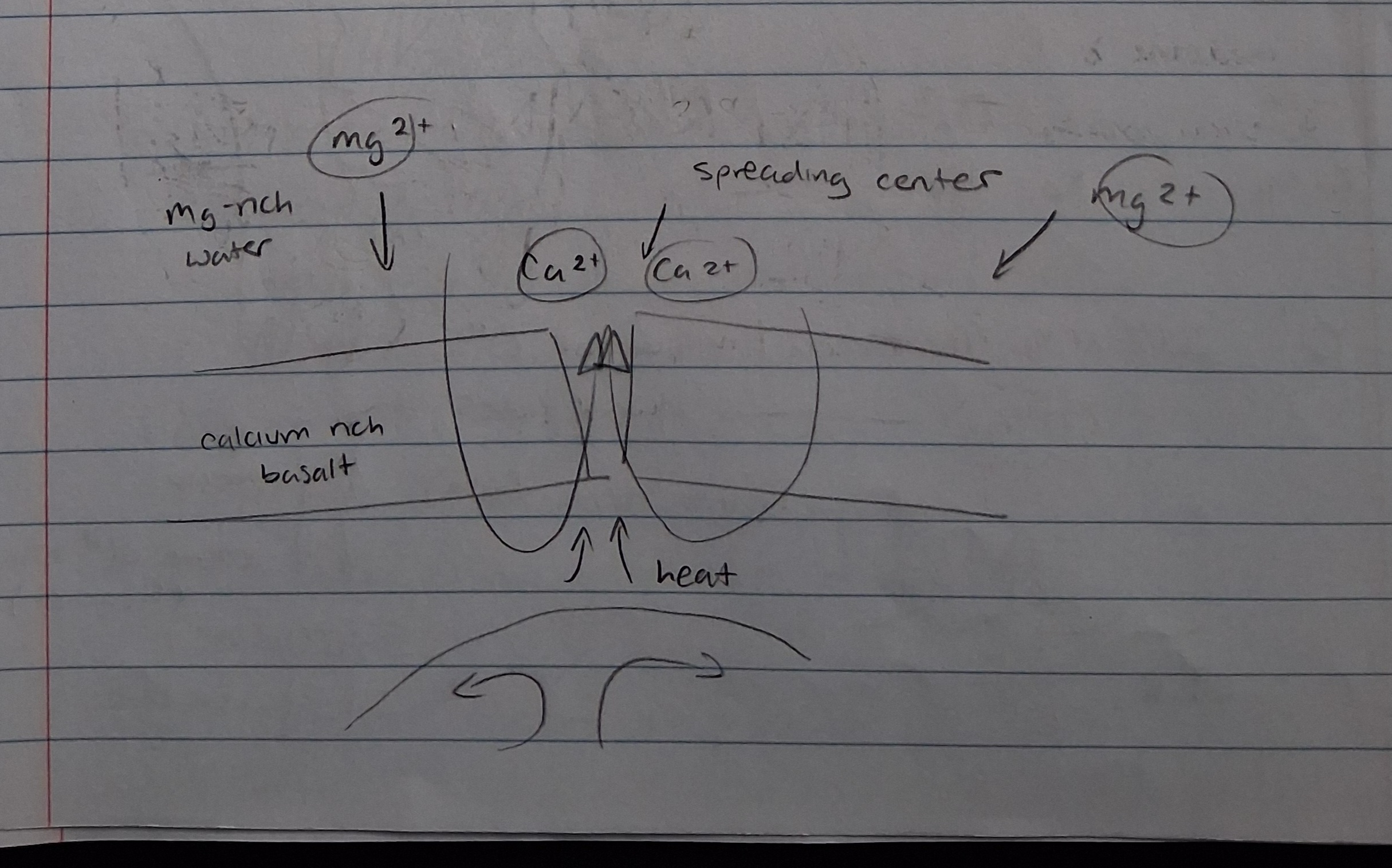
Fast vs. slow seafloor spreading rates (effect on Ca/Mg ratio)
Calcium to magnesium ratio in water increases (more Ca)
Calcium to magnesium ratio in water decreases (less Ca)
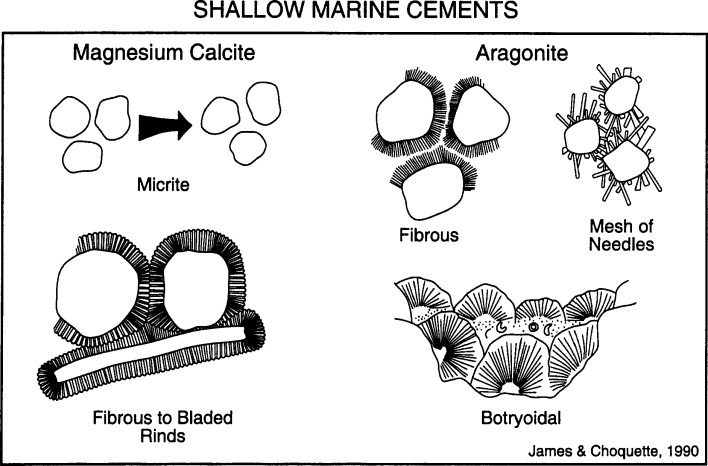
Shallow marine cements
Aragonite cements tend to form splays, acicular, botryoidal, and fibrous crustal structures (uncommon in ancient rock)
Magnesium-calcite cements tend to form stubby, bladed, and peloidal structures
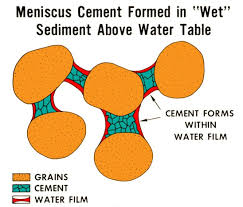
Meniscus cement
Weak cements occurring in vadose environments (beachrock) that connect the points of grains. Often seen as carbonate lining that encrusts rocks you might pick up on the beach
Crinoid columnals tend to form
calcite overgrowths; they are calcitic and so their original structure is often preserved in thin section
Vadose meteoric cements
Meniscus cement, vadose silt, micritic envelope, epitaxial
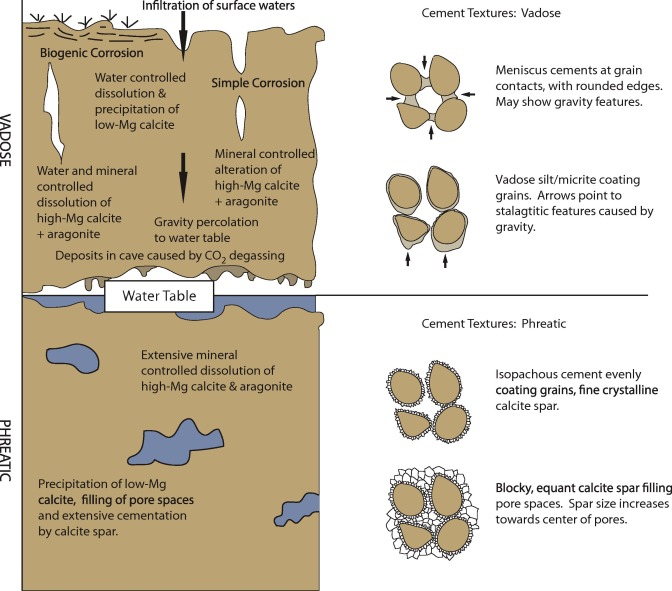
Phreatic meteoric cements
Isopachous, blocky, epitaxial

Calcite cements occur prior to
Major compaction of pore space (shallow burial, early diagenesis)
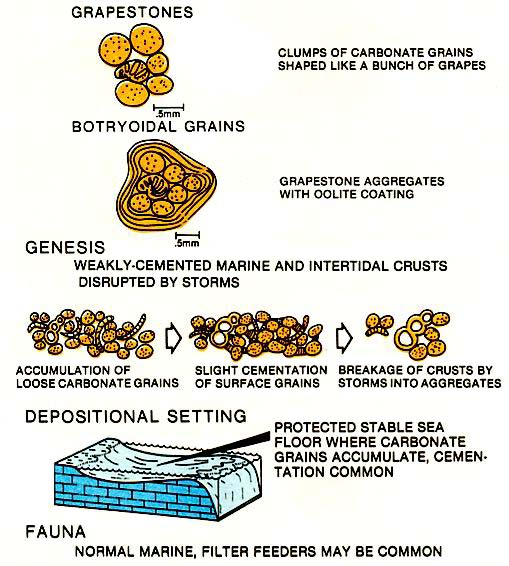
Grapestones
Lightly cemented together in point to point contact in the depositional environment. These grains are then broken up by storm movements into circular chunks
Micritized skeletal grains
Type of peloid; skeletal grains form micritic growths in calm, shallow water environments
Carbonate lithics
Form as a result of sea level drops, exposing a reef that terrestrial rivers then erode. As a result, you get breakup of carbonate (limestone) into lithic chunks. Can also happen in the event of a wave-formed sea cliff
common in recycled orogen
Primary porosity is present during
Pre-depositional and depositional processes
Secondary porosity is present during (primary porosity absent)
Post-depositional processes
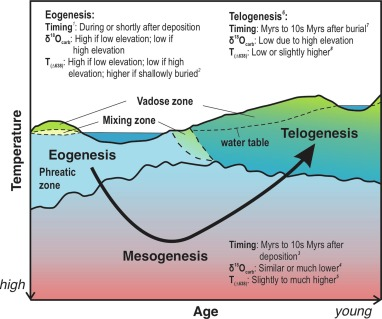
Eogenesis
Early burial; carbonates form at or before the deposition of the sediment itself (during deposition)
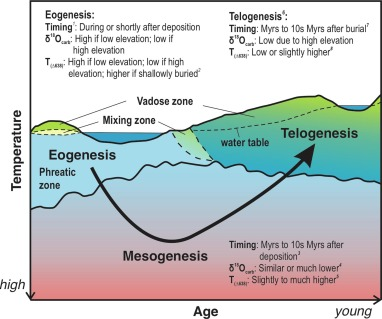
Mesogenesis
Deep burial; carbonates undergo mid and late diagenesis. Longest stage
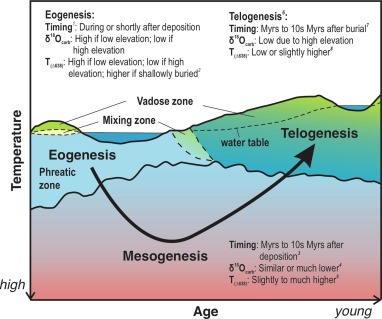
Telogenesis
Carbonates are uplifted after diagenesis and exposed to the meteoric zone
Carbonate porosity types
Fabric or non-fabric selective
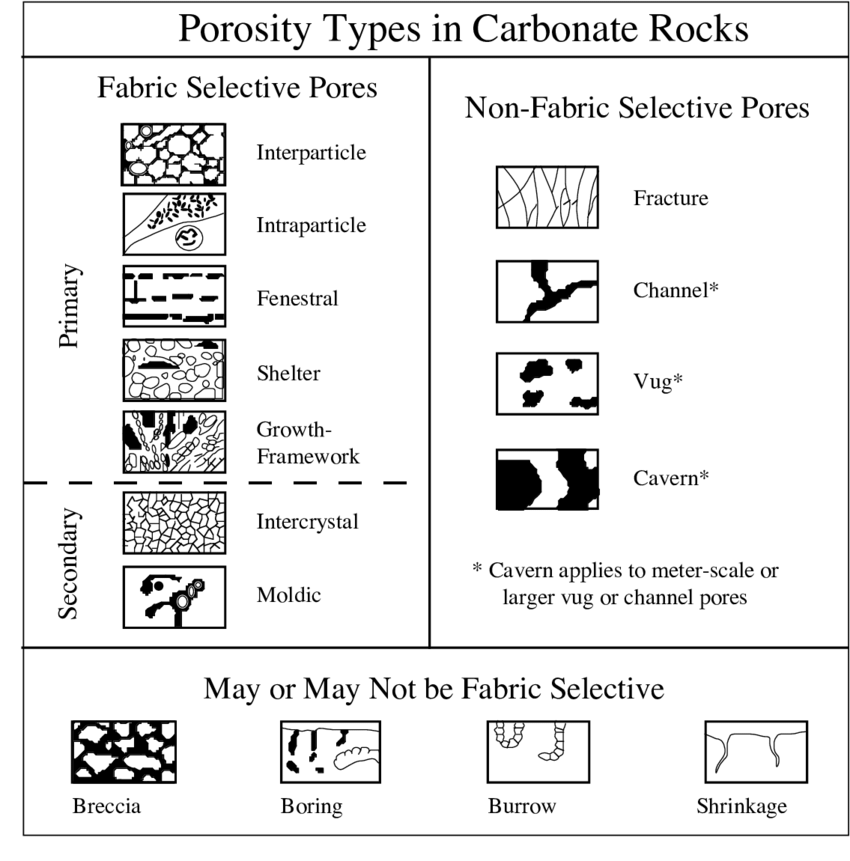
Moldic pore
Forms due to selective dissolution of a particular component (aragonite)
Stylolites
jagged, interlocking surface in a rock, most commonly found in limestone, that forms from pressure dissolution. Minerals that are insoluble, like clay or iron oxides, become concentrated along the surface while the more soluble minerals are dissolved, leaving a dark, visible seam that looks like a zig-zag suture
