Chapter 3: Energy, Catalysis, and Biosynthesis
1/25
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
26 Terms
metabolism
All of the chemical reactions that occur within an organism to survive, grow, and reproduce
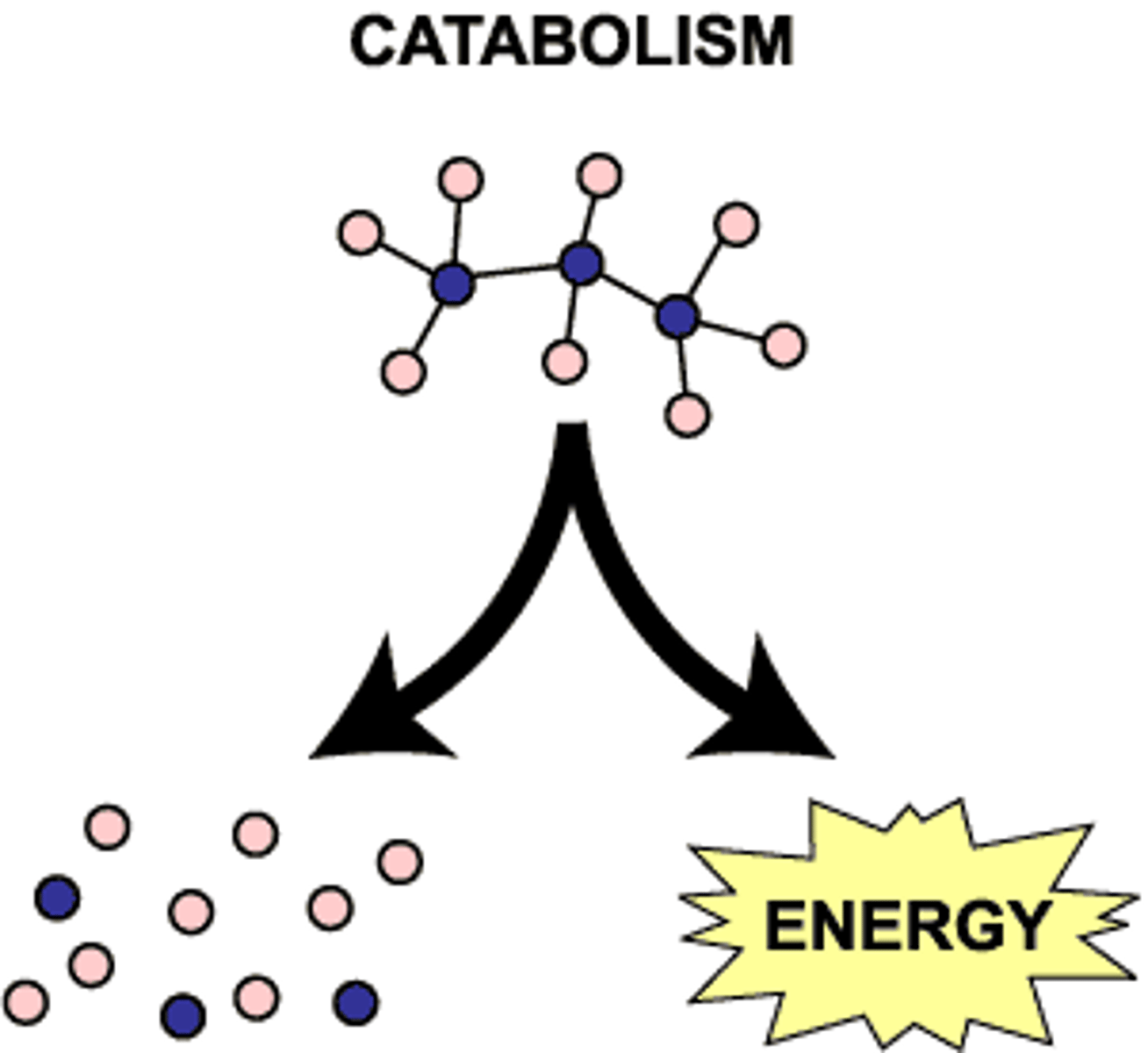
catabolism
Metabolic pathways that break down molecules, releasing energy.
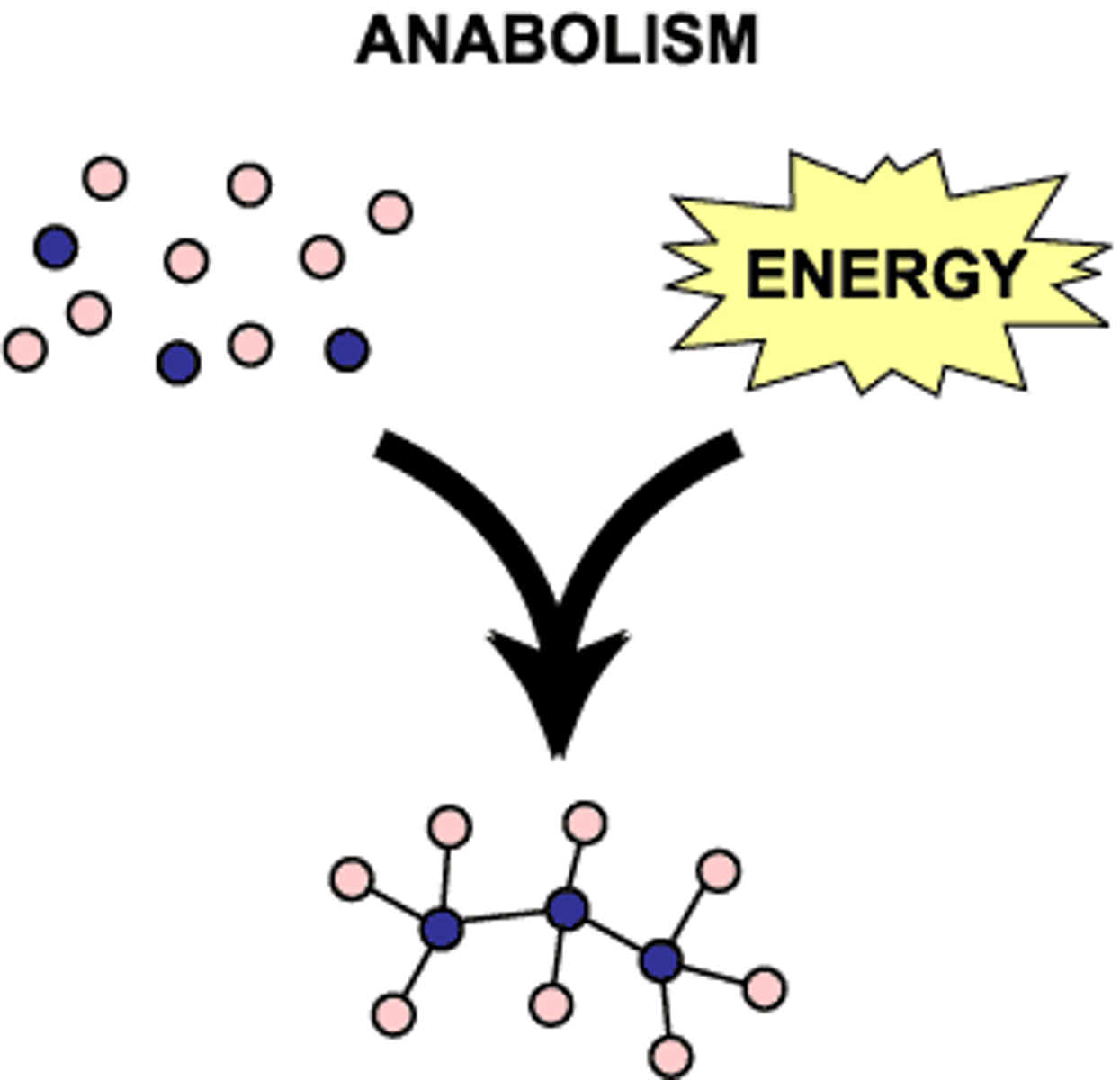
anabolism
Metabolic pathways that construct molecules, requiring energy.
food molecules are dissipated as
heat
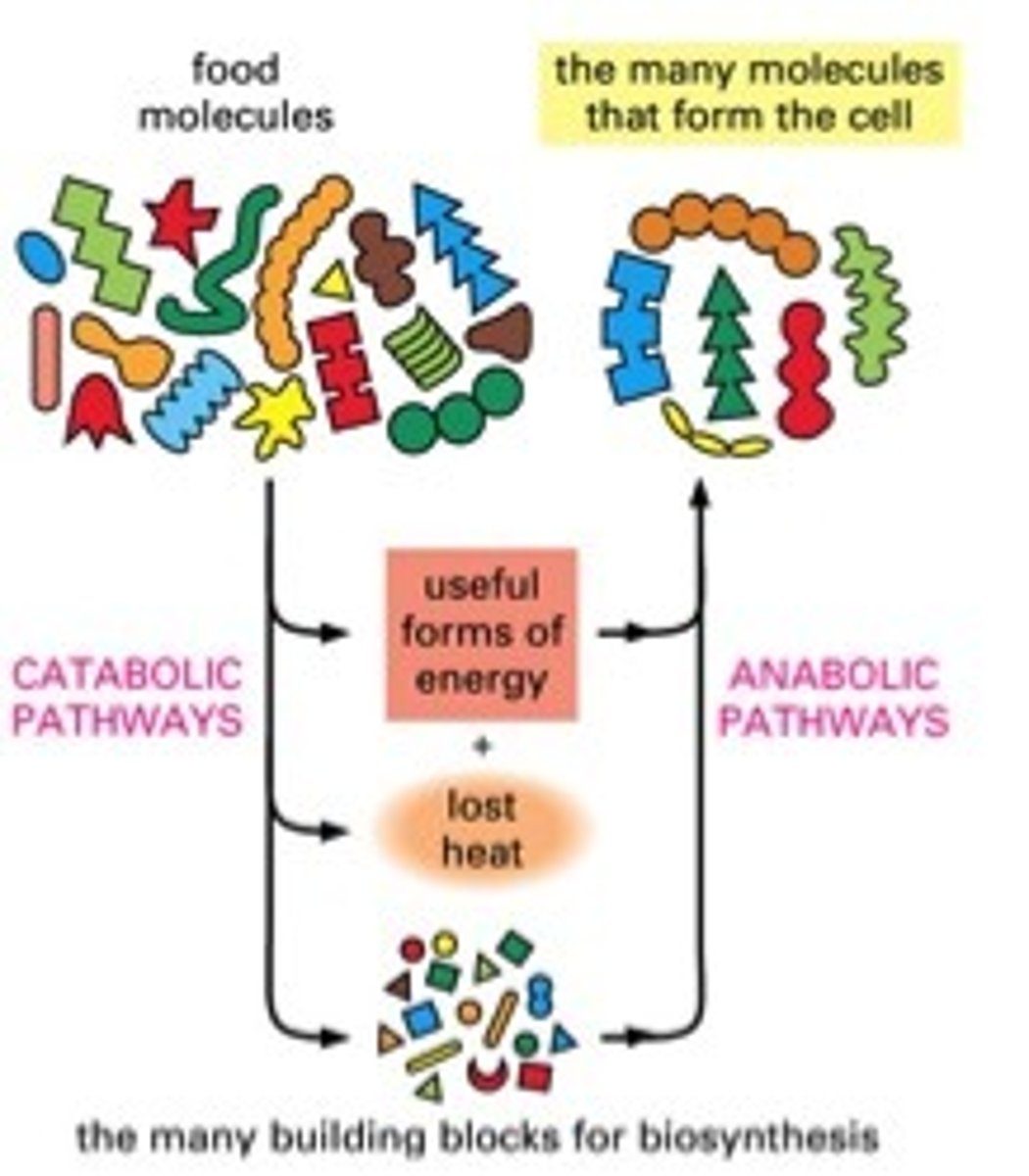
heat is a product of
energy conversion
biological order is made possible by
the release of heat energy from cells
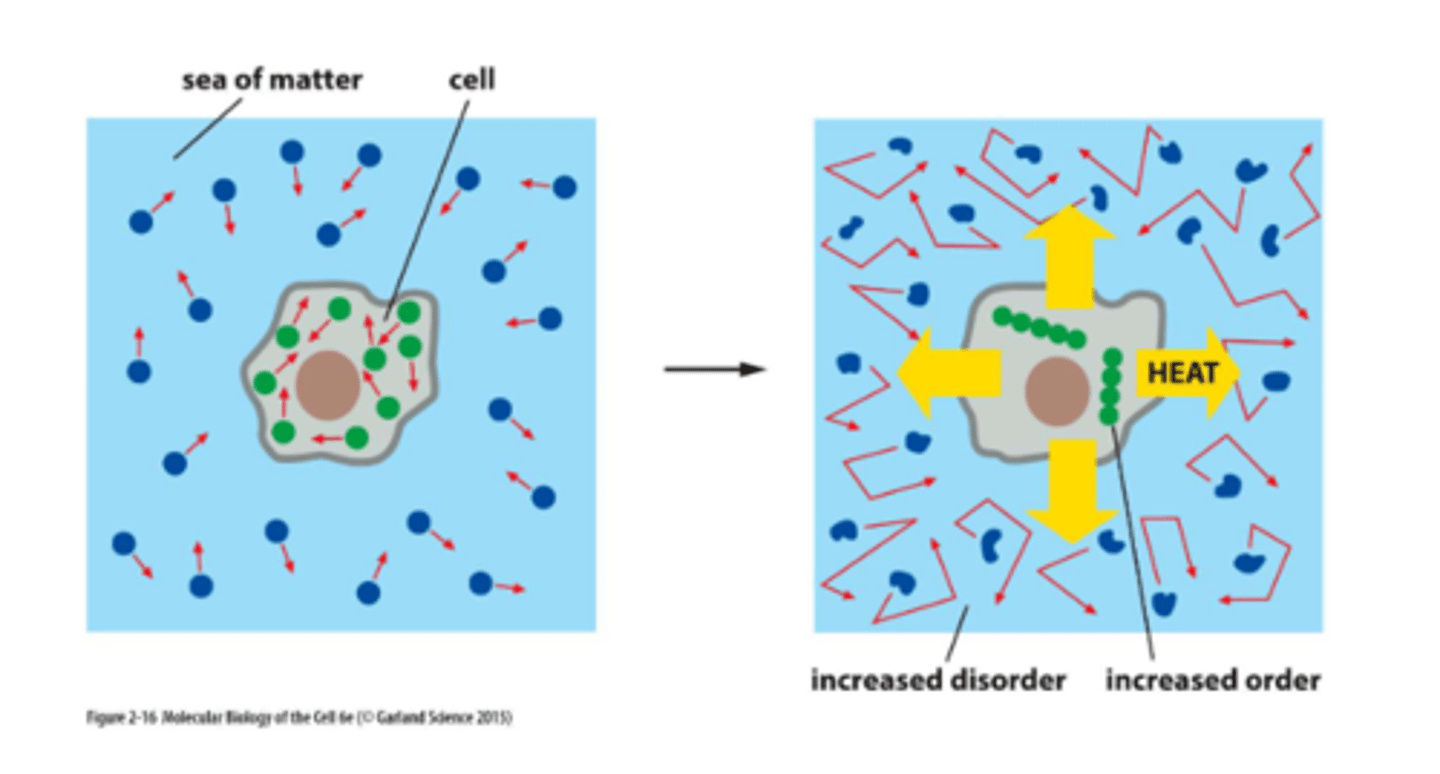
Second law of thermodynamics states that the
Every energy transfer or transformation increases the entropy of the universe.

movement toward disorder is
-spontaneous
-required input of energy to reverse it
entropy
A measure of disorder or randomness.
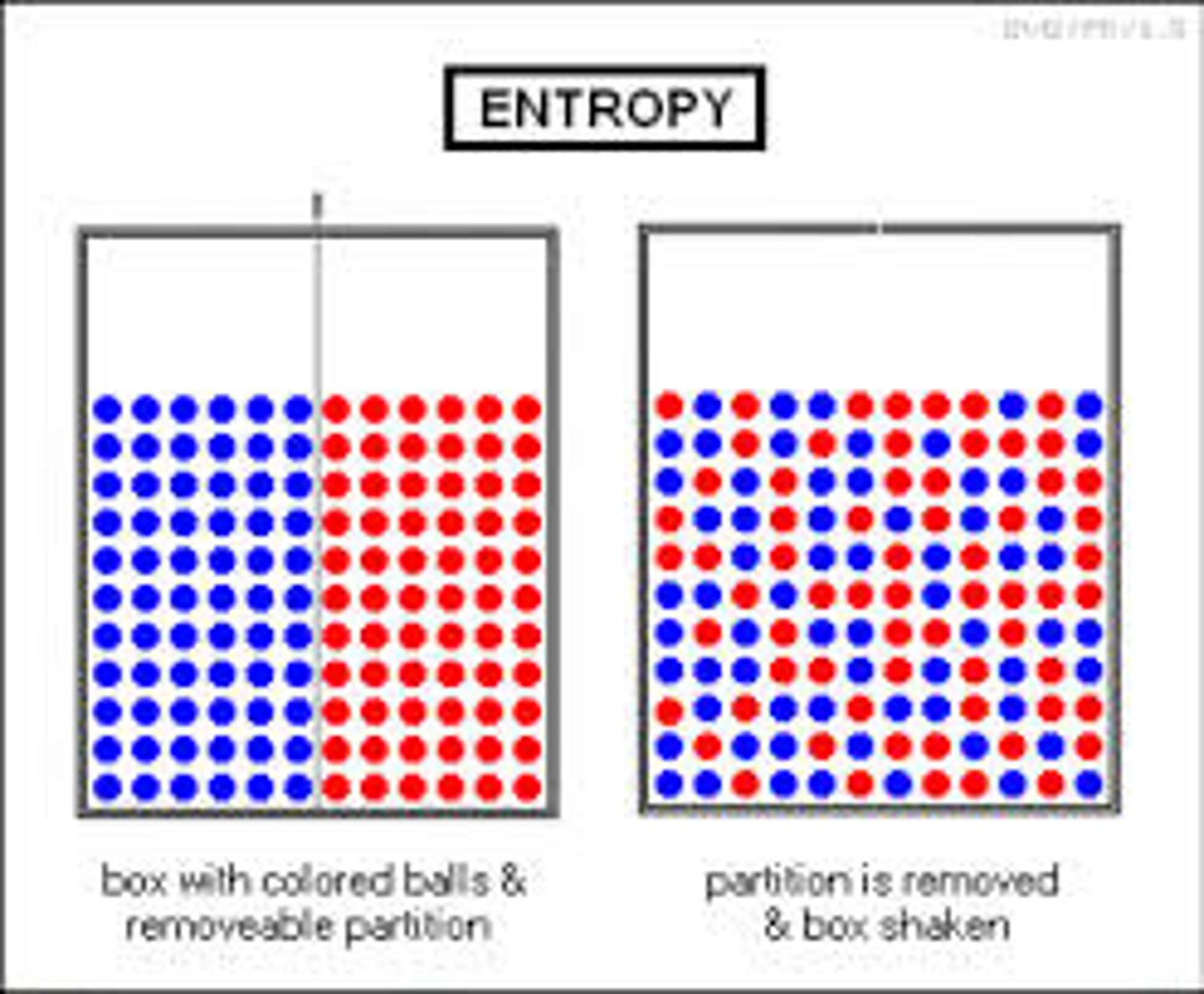
The greater the disorder the ________________ the entropy
greater
systems will change spontaneously towards
arrangements with greater entropy
the chemical rxns inside a cell must increase
the total entropy of the entire system: cell and enviornment
The first law of thermodynamics states that
energy cannot be created nor destroyed, the energy in the universe must always be the same
cells obtain energy by the
oxidization of organic molecules
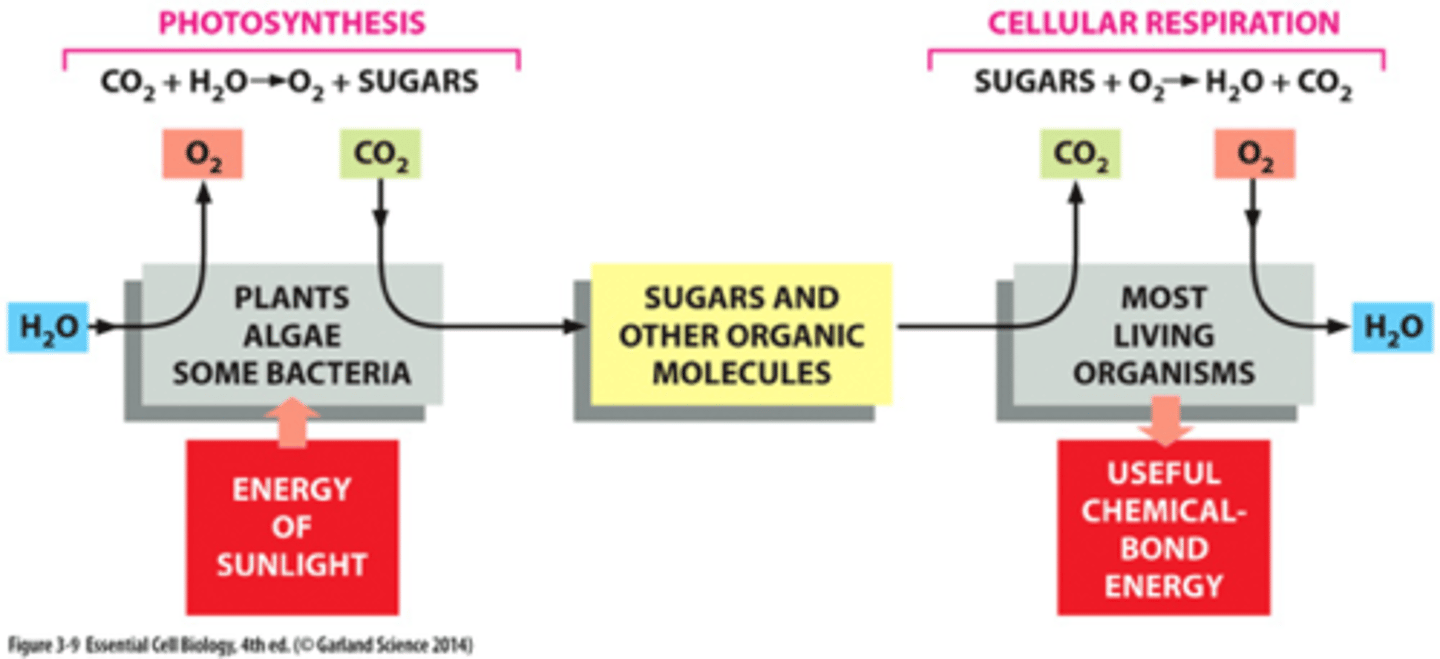
What is usually produced of the oxidization of organic molecules
CO2 and H2O (cell respiration)
biosphere
the collection of living things on earth
Carbon atoms are continuously being
cycled through the biosphere
electrons power
chemical reactions (oxidization and reduction)
oxidization
removal of electrons
reduction
addition of electrons
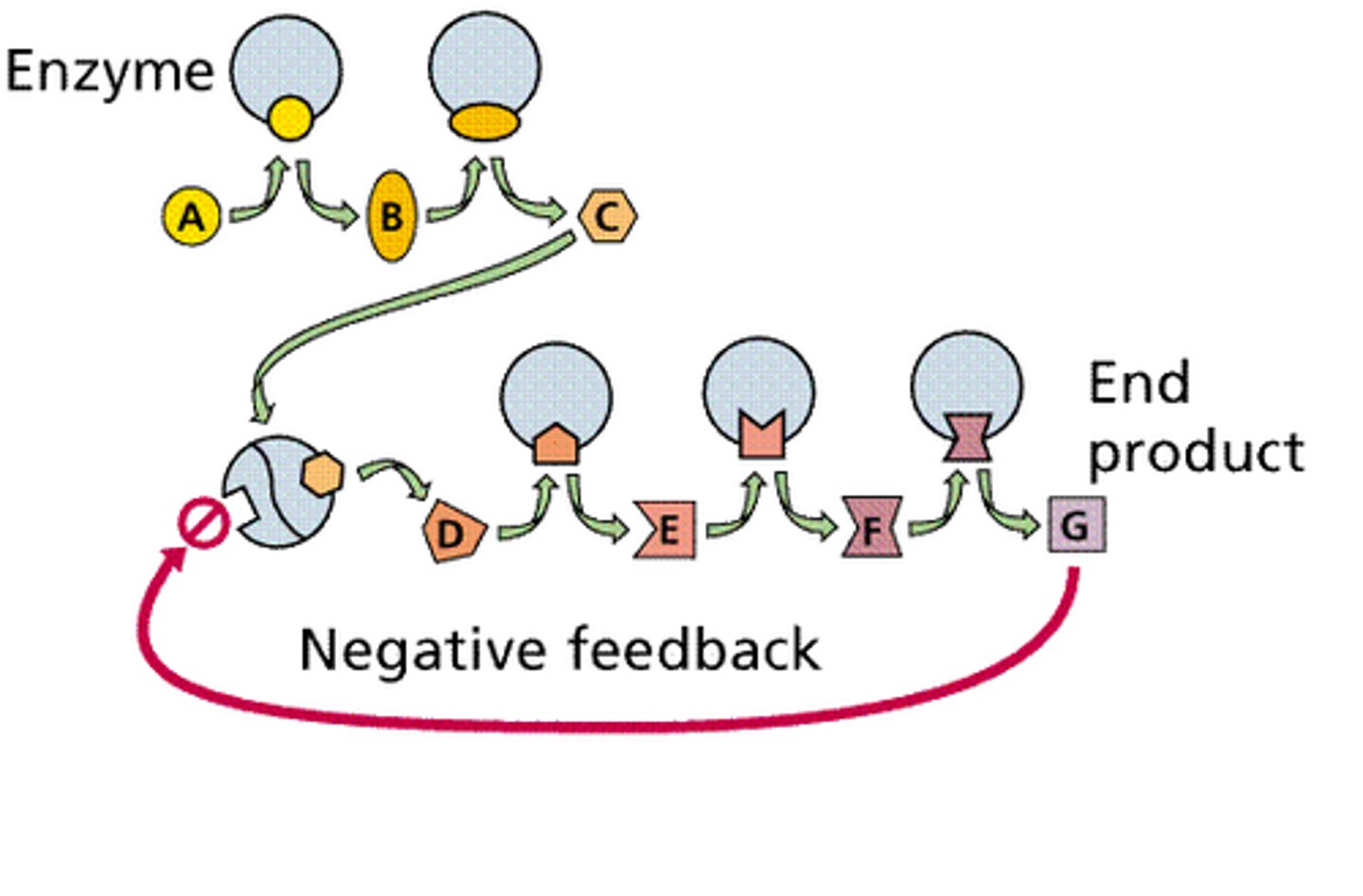
enzymes can convert substrates to
products
a chemical reaction can only proceed if it results in a net increase in disorder
delta G < 0
SPONTANEOUS,
- energy is released,
-energetically favorable
delta G > 0
-NONSPONTANEOUS,
-needs energy input
-energetically unfavorable
enzymes help create biological order by
coupling energetically unfavorable (Positive G) and energeticslly favorable (negative G) whole entire net is negative
chemical reactions tend to proceed until
equilibrium and cannot change it