3.1.3.7 forces between molecules
1/25
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
26 Terms
there are only intermolecular forces between what molecules?
covalently bonded molecules
covalent bonding leads to what?
the formation of giant (macromolecular) or simple molecules
how many types of intermolecular forces are there?
three
give the three types of intermolecular forces + give their relative strengths
weakest → strongest order :
Van der Waals forces between molecules
permanent dipole-dipole forces between molecules
hydrogen bonding between molecules
Van der Waals forces are present in what molecules?
they are present in all molecular substances
what does the relative strength of the intermolecular force depend on?
on the size of the molecule
how can you determine the type of intermolecular forces that hold the molecules together?
by determining if the molecule is polar/non-polar
what about a molecule tells you if its polar or not?
if its asymmetrical, its polar
if its symmetric, its non-polar
but how can you work out if a molecule is asymmetrical/symmetrical and so polar/non-polar?
add on partial charges on polar bonds
look to see if there’s an even distribution of the electron density in the molecule ie if the molecule is more 𝝳⁻ at one end
even distribution = symmetrical
explain what a temporary dipole is and how its formed
electrons are constantly moving + so the charge can change distribution at any time
this means one side of a molecule could suddenly become more negative than another if the electrons have moved closer to that side
this is called a temporary dipole
when does a dipole occur?
when there is an uneven distribution of electron density in the molecule
what is the effect of temporary dipoles?
it causes Van Der Waals forces to form
explain how VdW forces arise
a temporary dipole in one molecule induces a dipole in another molecule
there is an attraction between a 𝝳⁺ on one molecule and a 𝝳⁻ on an adjacent molecule
this is called the van der Waals intermolecular force
true or false? all simple molecules only have the VdW intermolecular force
false → all simple molecules do but some simple molecules have other intermolecular forces too
what 2 factors affect the strength of Van der Waals forces? + how?
Size/Mr of the molecule (bigger molecule, more VdW forces between molecules)
Surface area contact (more S.A. contact, more VdW forces between molecules)
→ more forces, the greater the strength
what effect does more VdW forces have on the molecule?
it results in higher melting and boiling points
permanent dipole-dipole forces occur between what type of molecules?
polar molecules
define what a polar bond is + what it results in
its where there is a difference in electronegativity between the atoms
there is then an attraction between a 𝝳⁻ on one molecule and a 𝝳⁺ on an adjacent molecule
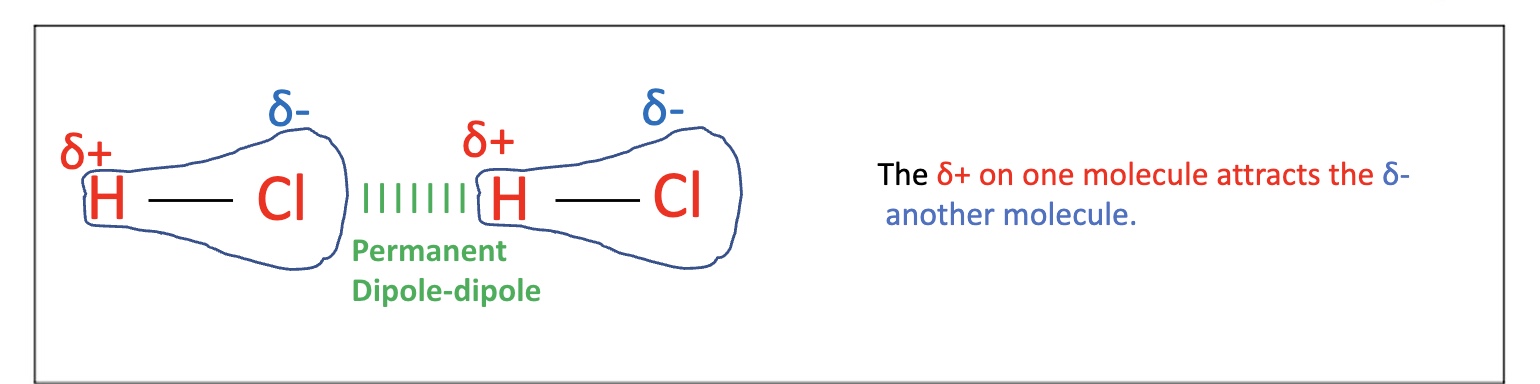
for example, explain why there is a permanent dipole-dipole between HCl molecules
there is a polar bond due to a difference in electronegativity between the H and the Cl
there is an attraction between a 𝝳⁻Cl on one molecule and a 𝝳⁺H on an adjacent molecule
hydrogen bonding is similar to permanent dipole-dipole but how does it differ from it?
it only occurs between polar molecules which have a hydrogen directly bonded to oxygen, nitogen, or fluorine
why are only O, N and F involved in hydrogen bonding?
because they are the most electronegative atoms
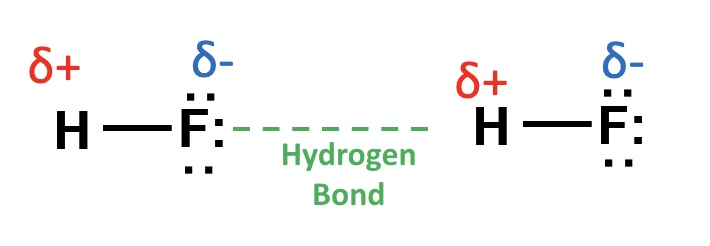
explain how hydrogen bonding arrises + what it involves
O,N and F are very electronegative
so there is a big difference in electronegativity between these atoms + the hydrogen
as a result, they pull the pair of electrons in the bond very strongly towards themselves
this leaves the hydrogen nucleus very 𝝳⁺
a lone pair of electrons on a neighbouring molecule containing oxygen, fluorine or nitrogen is then attached to the hydrogen
although its called hydrogen bonding is not…
a bond → its a force between two molecules
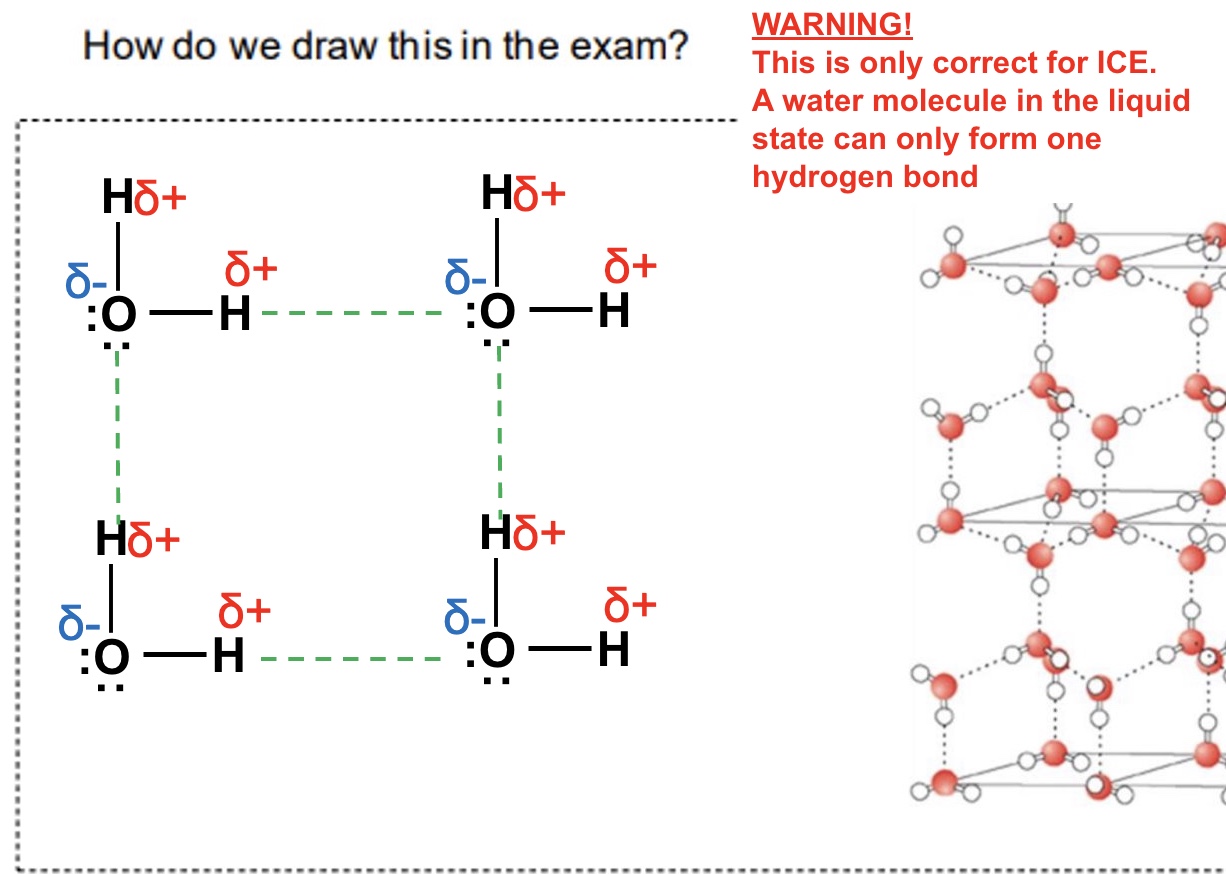
state the 4 features you must add to a hydrogen bonding diagram
partial charges on all atoms
all lone pairs clearly shown
hydrogen bond shown clearly between the lone pair + the 𝝳⁺ H on the other molecule
X : - - - - H — X must be linear
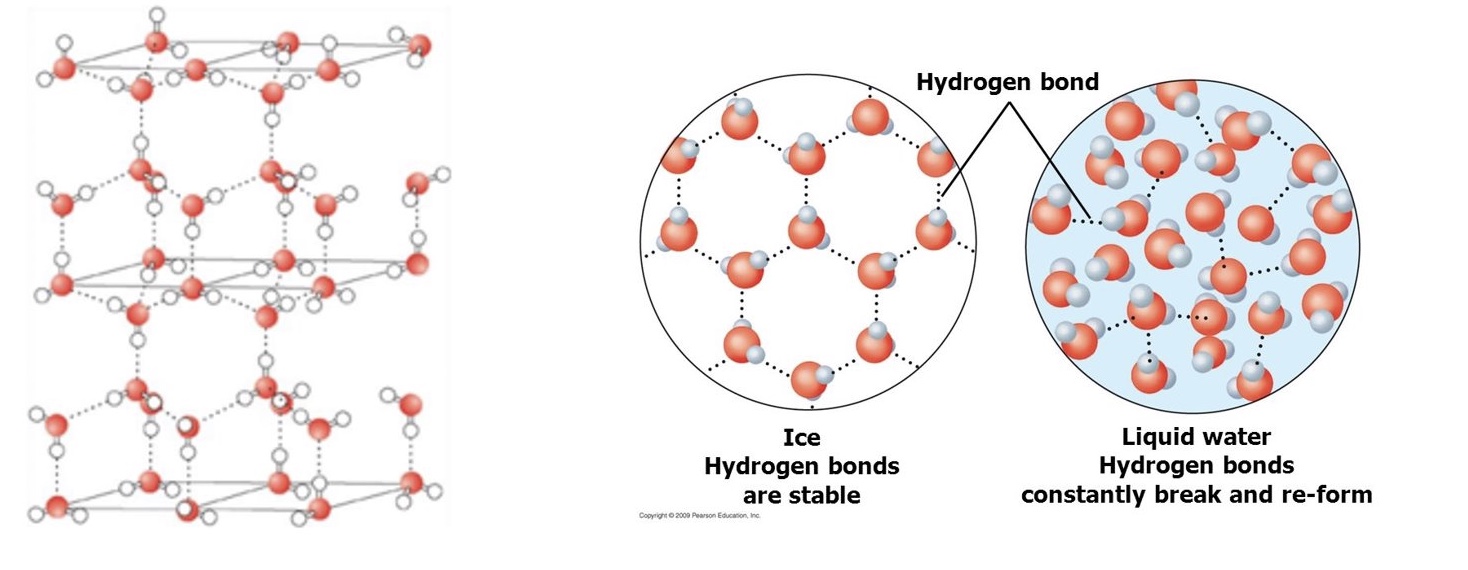
explain why ice is less dense than liquid water (applications of hydrogen bonding)
ice is less dense meaning that there are fewer particles of water per unit of volume in the solid state than the liquid state
this is because in ice, the water molecules are further apart than in liquid water due to hydrogen bonding
this means that there are more gaps/spaces between the molecules
how do we draw ice showing its hydrogen bonding?