Protein Synthesis
1/52
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
53 Terms
gene
a sequence of nucleotide bases in a DNA molecule that codes for the production of a specific sequence of amino acids, that in turn make up a specific polypeptide (protein)
where does transcription occur?
nucleus
Transcription (step by step)
Part of a DNA molecule unwinds
Hydrogen bonds between the complementary base pairs break
This exposes the gene to be transcribed
A complimentary copy of the code from the gene is made from mRNA
This reaction is catalysed by RNA polymerase
Free activated RNA nucleotides pair up, via hydrogen bonds, with their complementary bases on the exposed strand of the DNA molecule
The sugar-phosphate groups of these RNA nucleotides are bonded
catalysed by enzyme RNA polymerase
Sugar-phosphate backbone of the mRNA molecule formed
When the gene has been transcribed and the mRNA molecule is complete, the hydrogen bonds between the mRNA and DNA strands break
double-stranded DNA molecule reforms
The mRNA molecule then leaves the nucleus via a pore in the nuclear envelope
antisense/template strand
the strand that is transcribed
sense/non-template strand
the strand which is not transcribed
In what direction does the RNA Polymerase move?
3’ to 5’ direction
In what direction does the mRNA molecule grow
5’ to 3’ direction
Translation (step by step)
mRNA attaches to a ribosome
free tRNA molecules bind with their specific amino acids
the anticodon on each tRNA molecule pairs with the complimentary codon on the mRNA
Two tRNA molecules fit onto the ribosome at any one time
a peptide bond is formed between adjacent amino acids
continues until stop codon is reached
Where does translation occur?
Cytoplasm
anticodon
triplet of unpaired bases at one end
amino acid can attach
What is the effect of changing triplet code on a protein?
A different triplet code is produced as a triplet is substituted
À mutation could change one of the amino acids
This alters the bonds between R groups
Change in tertiary structure
Protein no longer carriers out (name specific function)
Codon
Sequence of three bases on mRNA
Triplet code
Sequence of three bases on DNA.
start codon
AUG
Which amino acid does the start codon code for
methionine
The three main components of the genetic code
non-overlapping
degenerate
universal
non-overlapping genetic code
each base is only read once
adjacent codons do not overlap
degenerate genetic code
multiple codons can code for the same amino acids
limits the effect of mutations
How many different codons are possible?
43 = 64
universal genetic code
almost every organism uses the same genetic code
enables genetic engineering
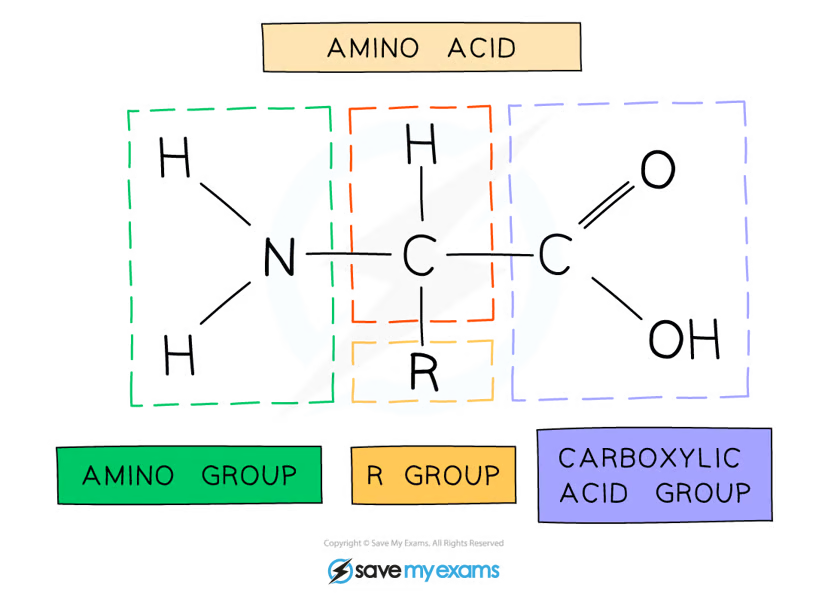
protein definition
polymer of amino acids
General structure of amino acids
amine group - NH2
carboxylic acid group -COOH
hydrogen atom
R group
Peptide bonds
OH lost from carboxylic group of one amino acid
H lost from amine group of one amino acid
remaining carbon atom on first amino acid bonds to nitrogen of second amino acid
condensation reaction
water released
Breaking down of peptide bonds
hydrolysis reaction
addition of water breaks peptide bonds
The four levels of protein structure
Primary
Secondary
Tertiary
Quaternary
Primary structure
sequence of amino acids bonded by covalent peptide bonds
determined by DNA
specific to each protein
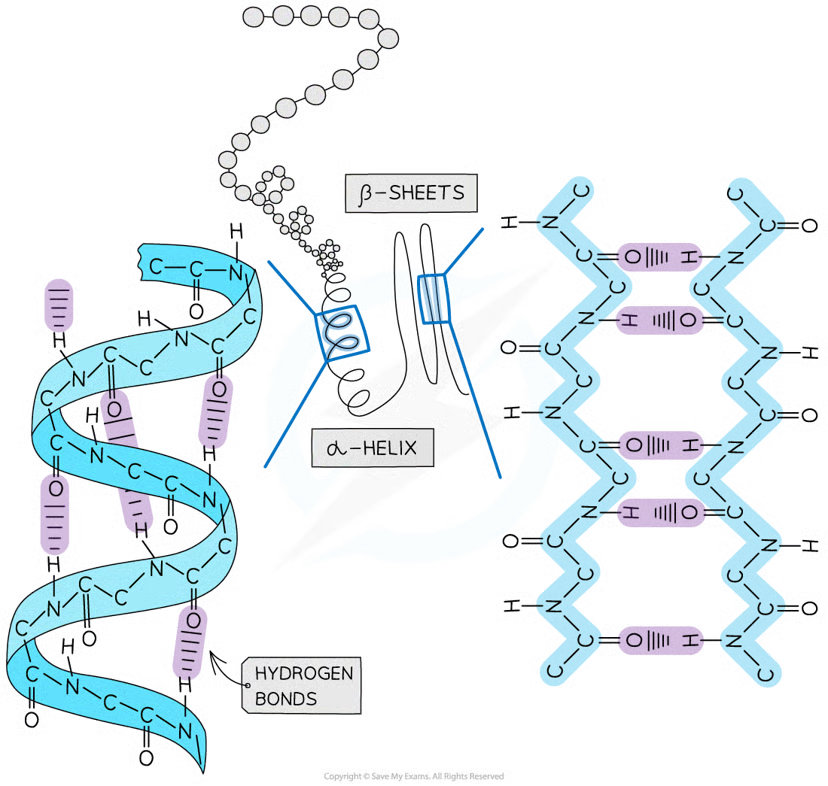
Secondary structure types
alpha helix
beta pleated sheets
How is the Secondary structure formed?
the weak negatively charged nitrogen and oxygen atoms interact with the weak positively charged hydrogen atoms to form hydrogen bonds
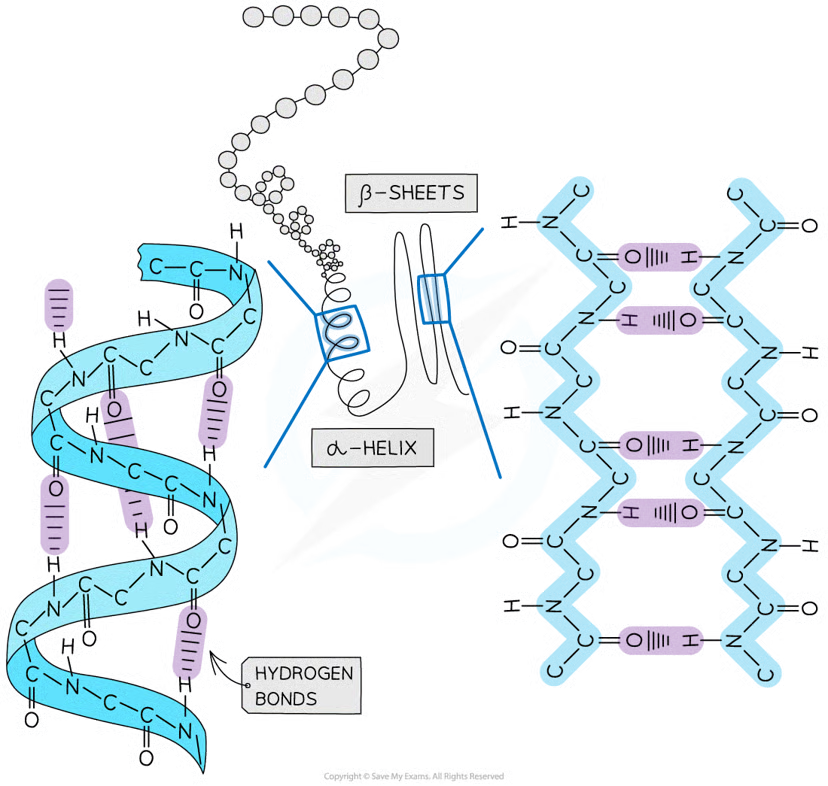
alpha helix
occurs when the hydrogen bonds form between every fourth peptide bond (between the oxygen of the carboxyl group and the hydrogen of the amine group)
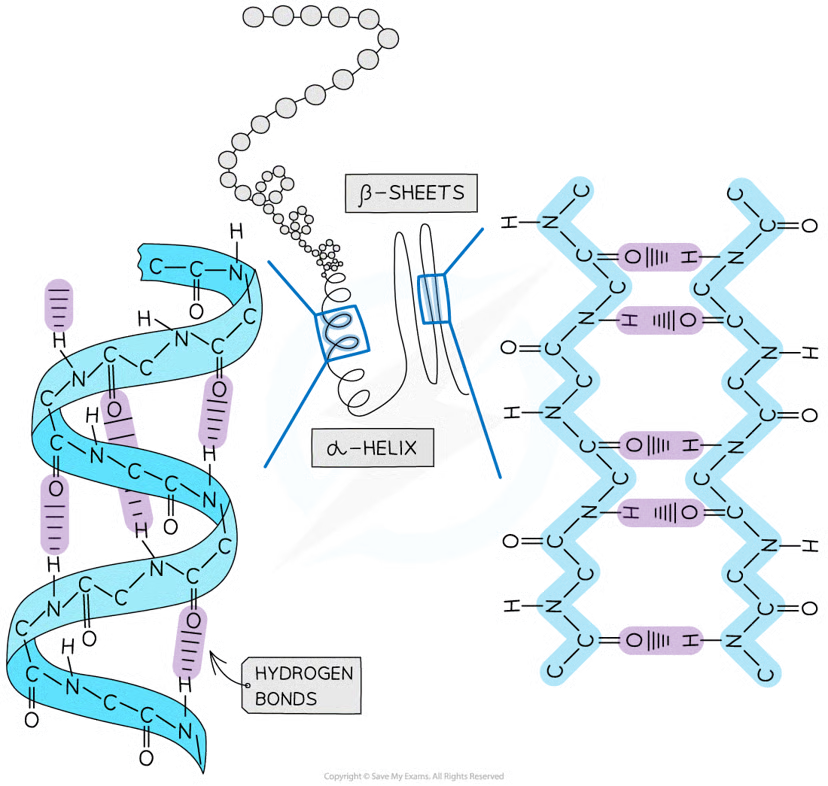
beta pleated sheet
the protein folds so that two parts of the polypeptide chain are parallel to each other enabling hydrogen bonds to form between parallel peptide bonds
What is the Tertiary Structure
bonds forming between R groups, hold together further folding of protein:
Hydrogen (these are between R groups)
Disulphide (only occurs between cysteine amino acids)
Ionic (occurs between charged R groups)
Weak hydrophobic interactions (between non-polar R groups)
What is quaternary structure?
multiple alpha helix and beta pleated sheets bonded together
How does incorrect folding effect the function of a protein?
tertiary structure altered
Active site changed
No enzyme-substrate complexes formed
Unable to catalyse reaction
globular protein main 2 features
compact
roughly spherical
How do globular proteins form a spherical shape?
Non-polar hydrophobic R groups are orientated towards the centre of the protein away from the aqueous surroundings
Their polar hydrophilic R groups orientate themselves on the outside of the protein
folding due to interactions between R groups results in shape
prosthetic group
Non-protein part of a protein molecule
globular protein function
soluble
molecules surround polar hydrophilic R groups
easily transported around organisms
haemoglobin structure
quaternary structure
4 subunits: 2 alpha globins, 2 beta globins
each subunit has a prosthetic haem group
What bonds hold together the four globin subunits in haemoglobin?
disulphide bonds
How does sickle cell anaemia come about?
base substitution results in the amino acid valine (non-polar) replacing glutamic acid (polar)
makes haemoglobin less soluble
How does haemoglobin carry oxygen?
haem group contains iron II
reversibly combines with oxygen molecule to form oxyhaemoglobin
each subunit can carry one oxygen molecules
How does haemoglobin transport oxygen?
haemoglobin is more soluble than oxygen in water
carried more efficiently with haemoglobin
as each oxygen molecule binds, quaternary structure is altered
haemoglobin has higher affinity for subsequent oxygen molecules
they bind more easily
due to haem group and iron II
Benefits of iron II
allows oxygen to bind reversibly
none of the amino acids in haemoglobin are well suited to binding with oxygen
fibrous protein structure
long strands of polypeptide with cross linkages due to hydrogen bonds
Fibrous protein features
insoluble in water
strong
How are fibrous proteins insoluble in water
large number of hydrophobic R groups
Fibrous protein examples
keratin
elastin
collagen
collagen structure
three polypeptide chains
held together by hydrogen bonds
forming a triple helix
covalent bonds form between R groups
in interacting triple helices
collagen fibrils
triple helices of held together by cross links
How are the collagen molecules positioned?
they are positioned the fibrils so that there are staggered ends
collagen fibre
many fibrils are arranged together
function of collagen
forms connective tissues
How does collagen have great tensile strength?
many hydrogen bonds within the triple helix structure
staggered ends of collagen molecules within fibrils provide strength