17. B Cell Development and B1
1/38
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
39 Terms
Where are naïve B cells located in lymph nodes, and what is their role before activation?
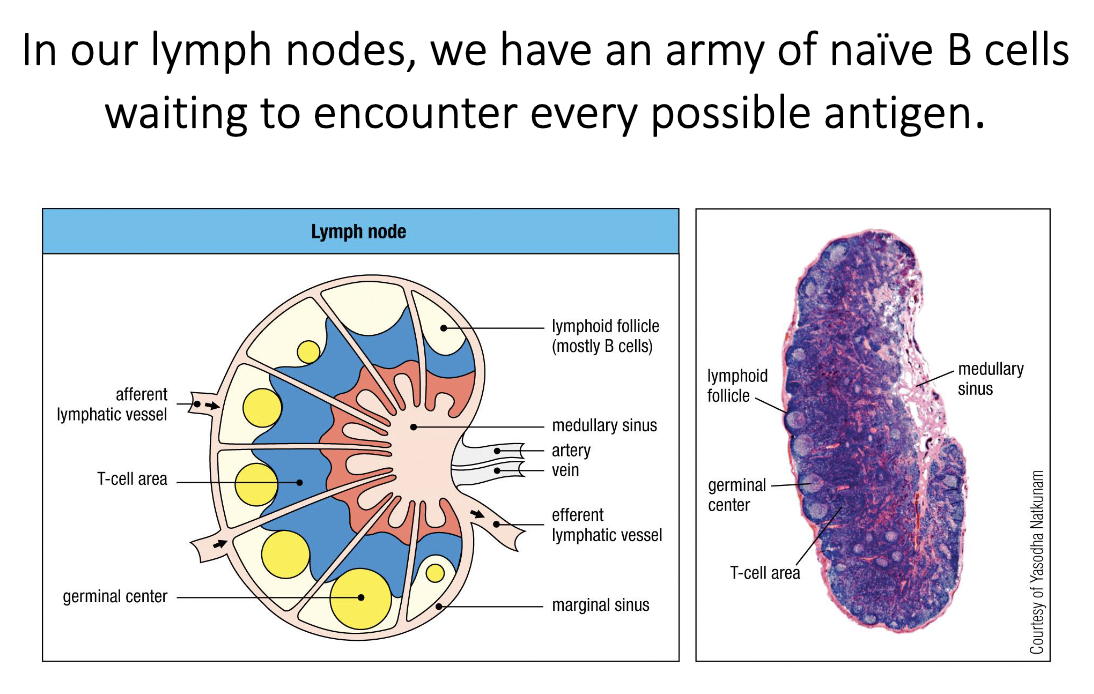
Lymph nodes contain an army of naïve B cells—not yet activated and not secreting antibody.
Their job is to wait for any potential antigen they might recognize.
They reside in B-cell follicles at the outer (peripheral) regions of the lymph node.
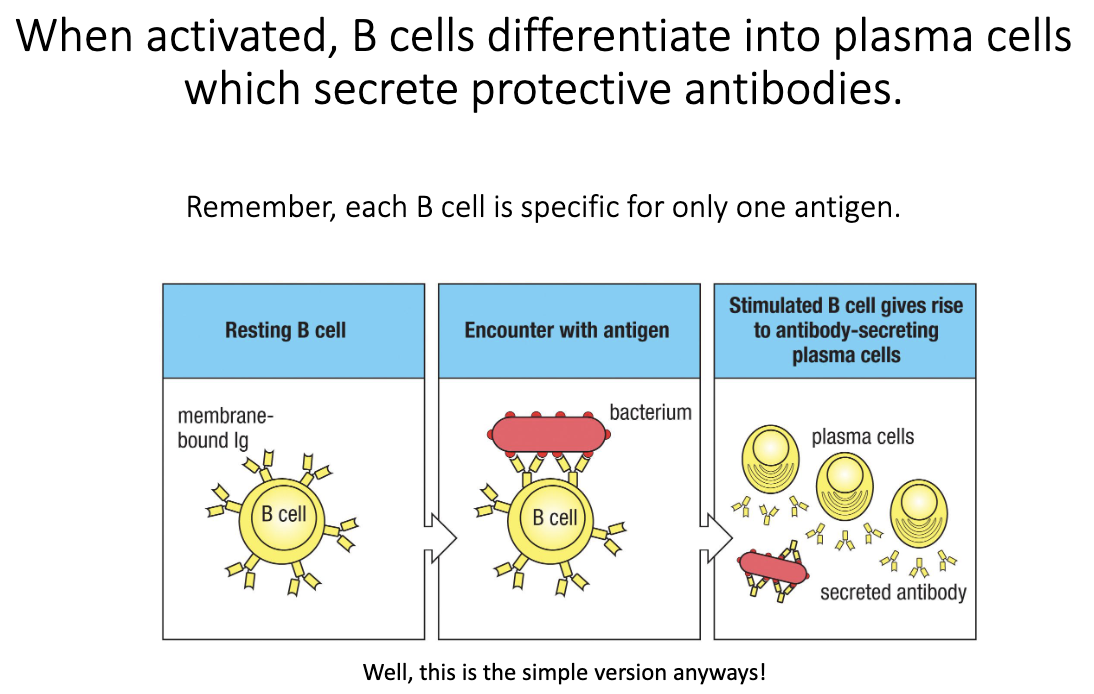
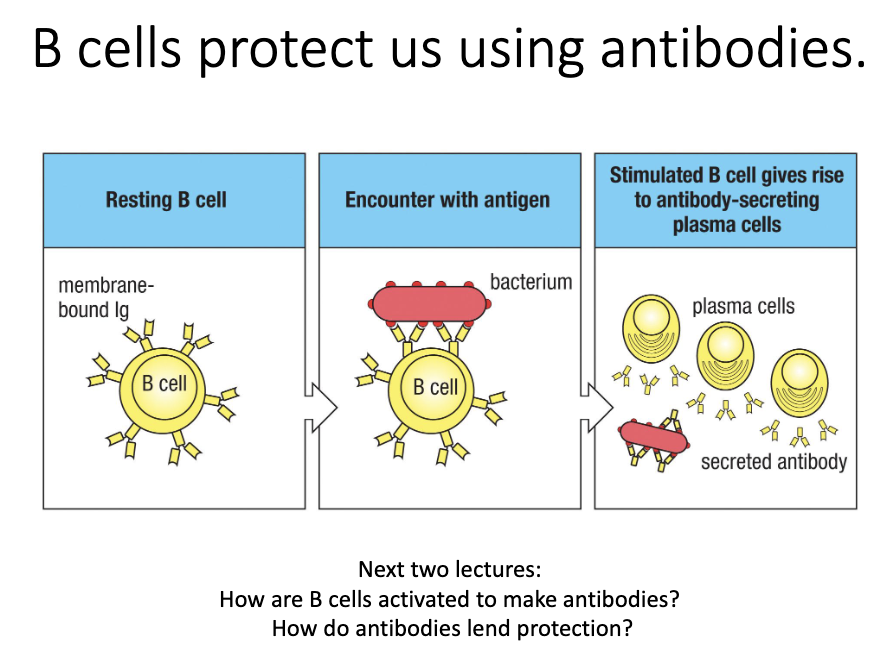
What happens when a B cell is activated?
Naïve B cell encounters its specific antigen.
Activation triggers differentiation into a plasma cell.
Plasma cells secrete large amounts of antibody.
These antibodies provide protective immunity.
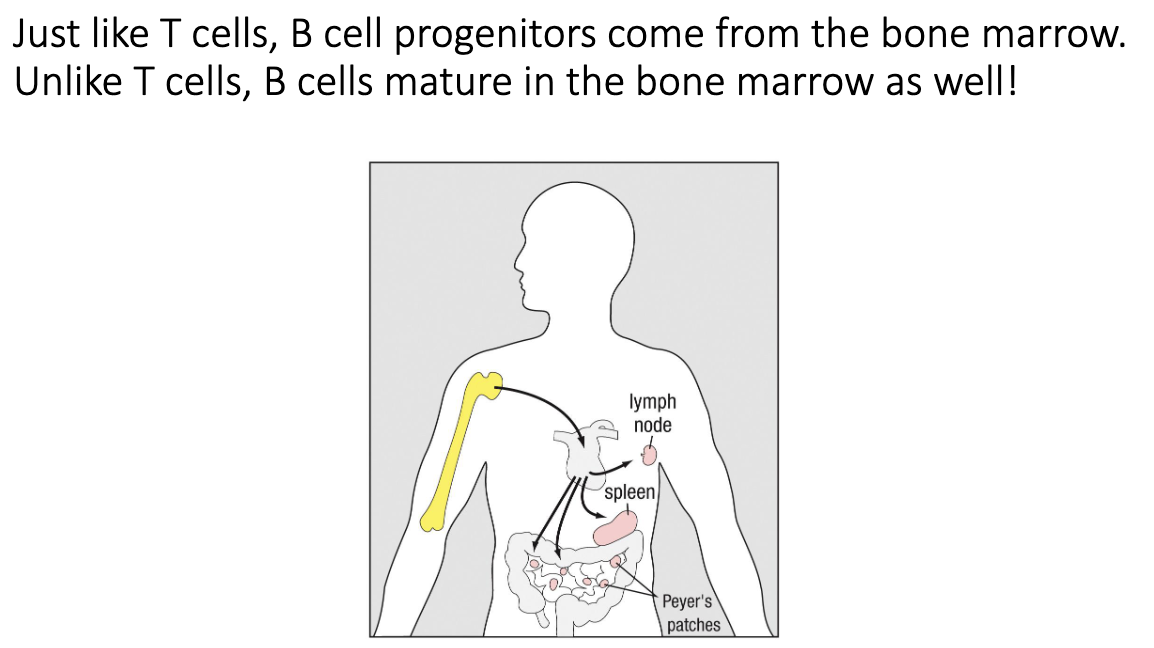
Where do B cells develop and mature?
B-cell progenitors come from the bone marrow.
Unlike T cells, B cells do not travel to the thymus.
All maturation happens in the bone marrow.
What does the “B” in B cell stand for, and how was it discovered?
“B” does NOT stand for bone marrow.
It stands for bursa of Fabricius—an organ in birds, not humans.
B cells were first discovered in chickens by removing the bursa and observing loss of B cells.
Early B-cell research wasn’t recognized as highly as T-cell work because the role of B cells was not yet understood.
We now know B cells make antibodies essential for protection.

Why must each B cell express only one B-cell receptor (BCR) specificity?
B cell must express one BCR sequence → one antigen specificity.
Prevents a single B cell from making antibodies with mixed specificities.
If a second specificity recognized self, infection-triggered activation could drive autoantibody production → dangerous self-attack.
B cells with self-reactive BCRs must be removed.
Functional B cells must also be able to traffic to lymph nodes and respond to activation signals.
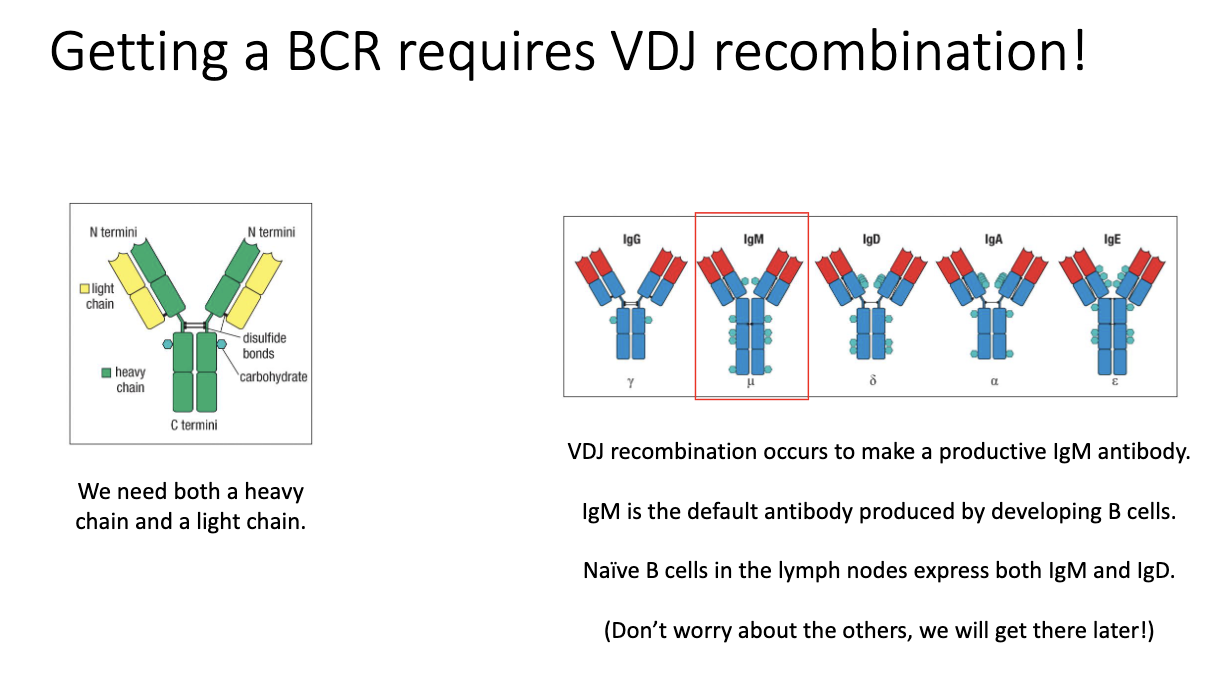
How do B cells generate their B-cell receptors, and what do naïve B cells express?
BCR/antibody generation uses VDJ recombination (like TCRs).
Requires rearrangement of heavy chain (analogous to TCR β) and light chain (analogous to TCR α).
Productive recombination yields an IgM antibody first.
Unique to B cells: they can later make multiple antibody isotypes.
Naïve B cells co-express IgM and IgD on their surface—the only stage that expresses both.
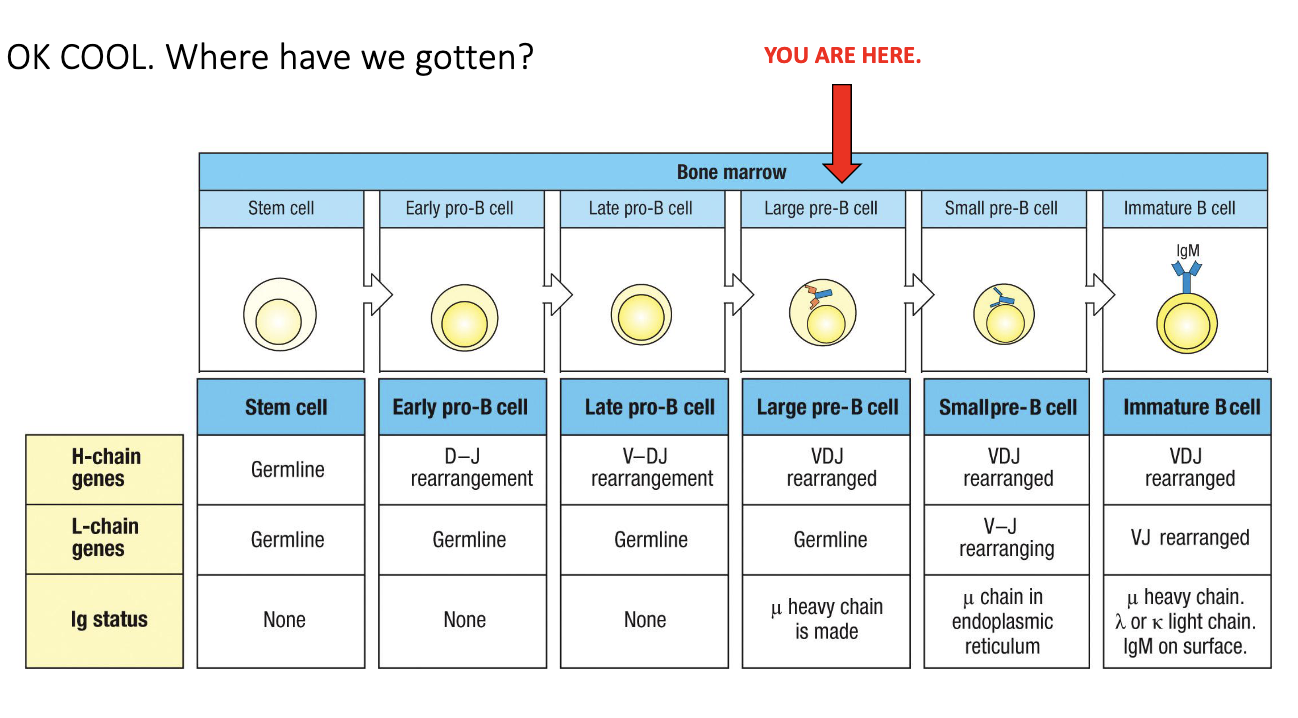
What is the overall structure of B-cell development?
Development occurs through multiple defined stages in the bone marrow.
Heavy-chain and light-chain genes undergo sequential rearrangements.
Each stage includes checkpoints determining whether the cell survives or dies.
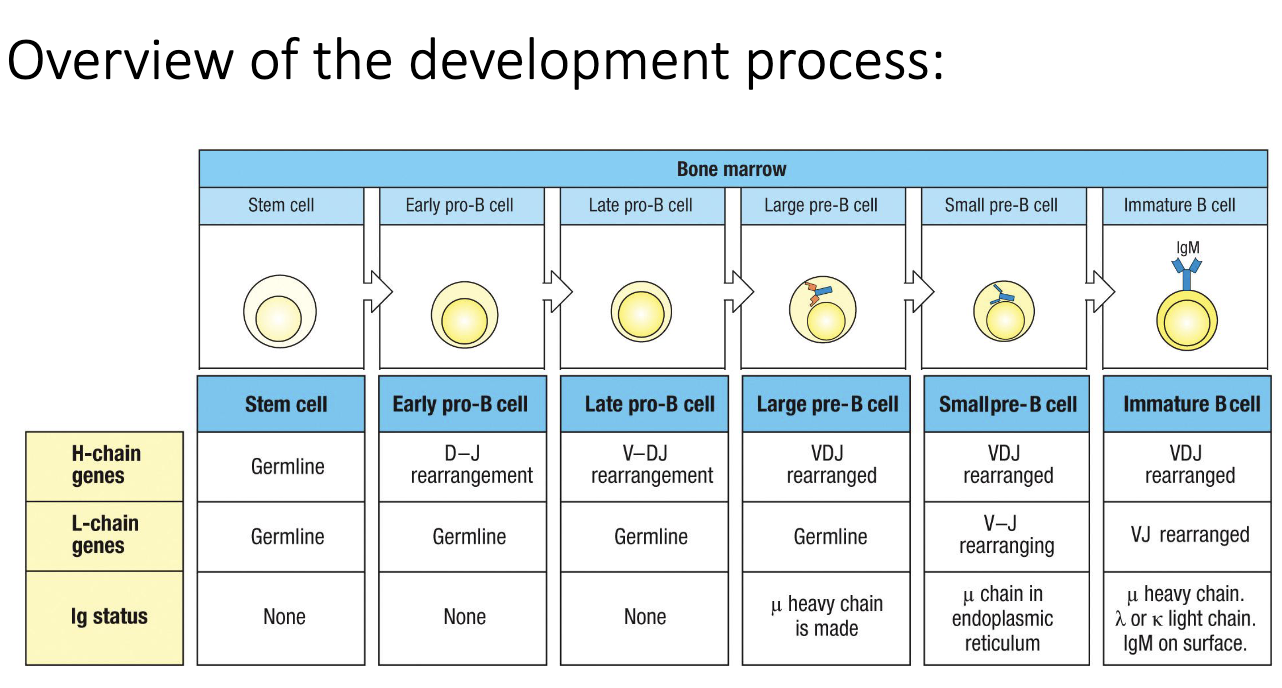
What does “germline” mean in the context of immunology and VDJ recombination?
Germline DNA = the unchanged sequence present in sperm or ovum.
Contains all V, D, and J segments before rearrangement.
Stem cells retain germline configuration so all descendant B cells can create unique rearrangements.
If the stem cell rearranged its DNA, all descendant cells would share the same VDJ → no diversity.
Early stem cells show no rearrangement in heavy or light chains.

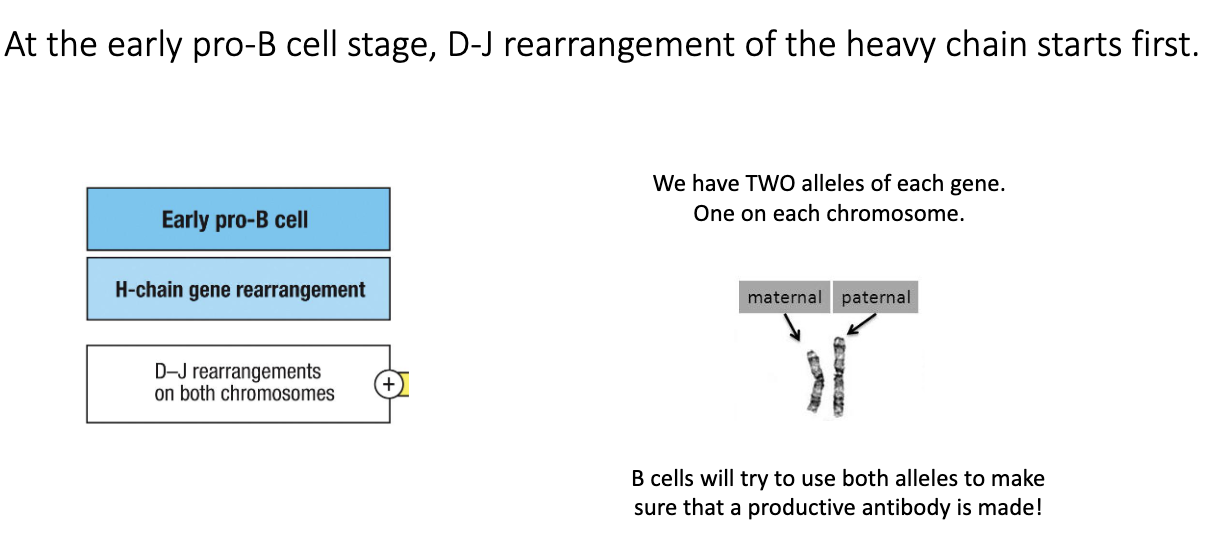
What happens during the early pro-B cell stage?
Early pro-B cells begin heavy-chain recombination.
First step: D–J joining on the heavy-chain locus.
B cells attempt D–J joining on both chromosomes (maternal + paternal).
Two heavy-chain loci = two chances for successful recombination.
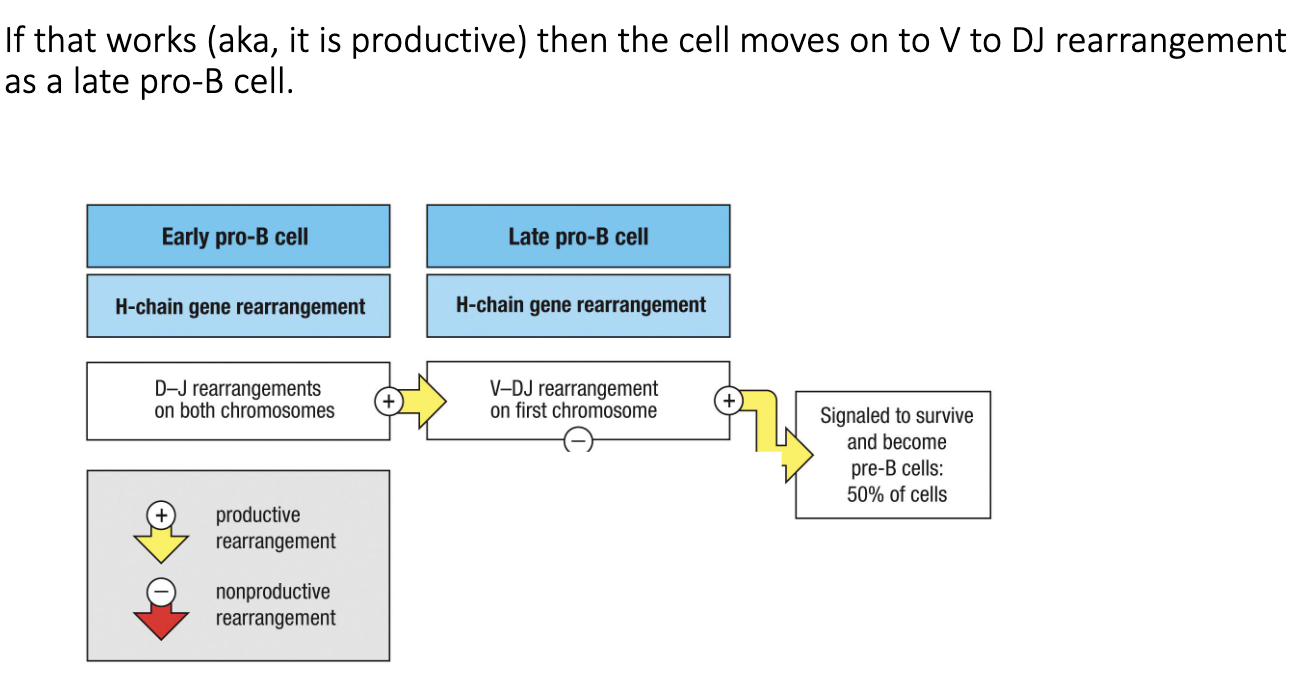
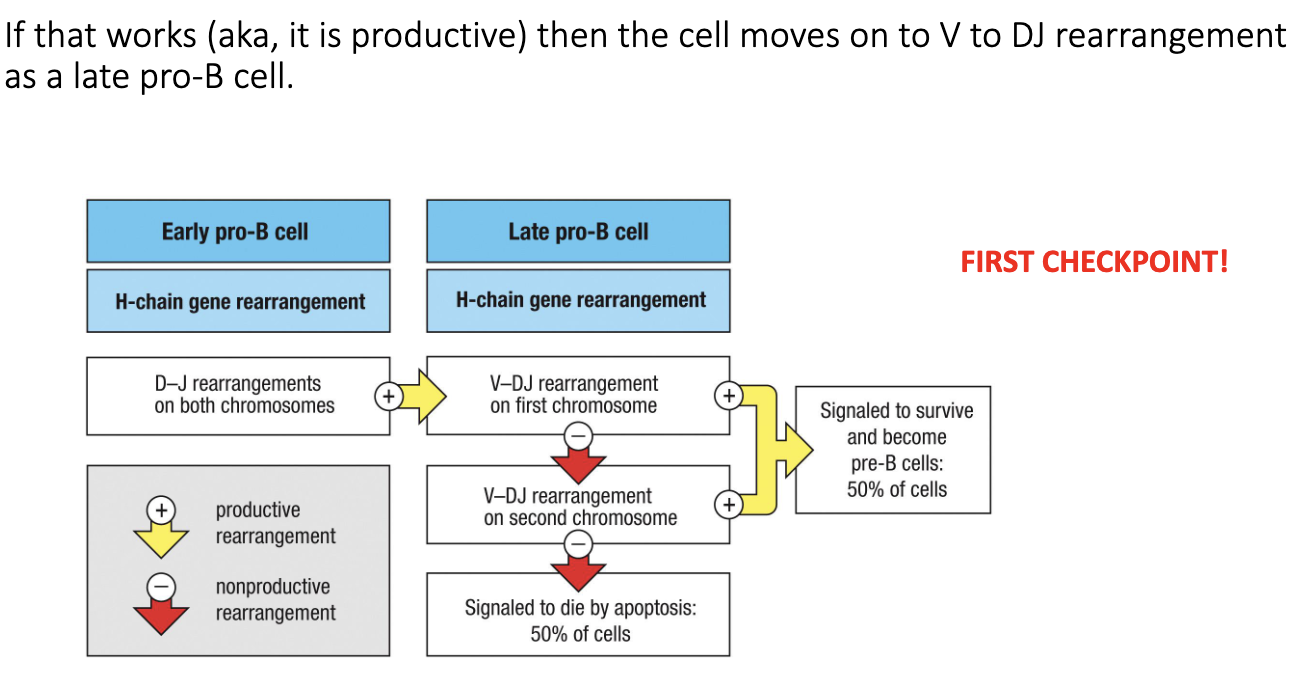
What occurs during the late pro-B cell stage?
After successful D–J joining → cell moves to late pro-B stage.
Next step: V → DJ recombination (full heavy-chain rearrangement).
Productive rearrangement = cell survival; non-productive = retry or die.
Only ~50% of cells successfully complete this step.
How does a B cell handle failed heavy-chain rearrangements?
If first chromosome’s V→DJ rearrangement fails, the cell tries the second chromosome.
If the second attempt also fails → apoptosis.
This system ensures each B cell tries to produce one functional heavy chain, while preventing survival of nonfunctional cells.
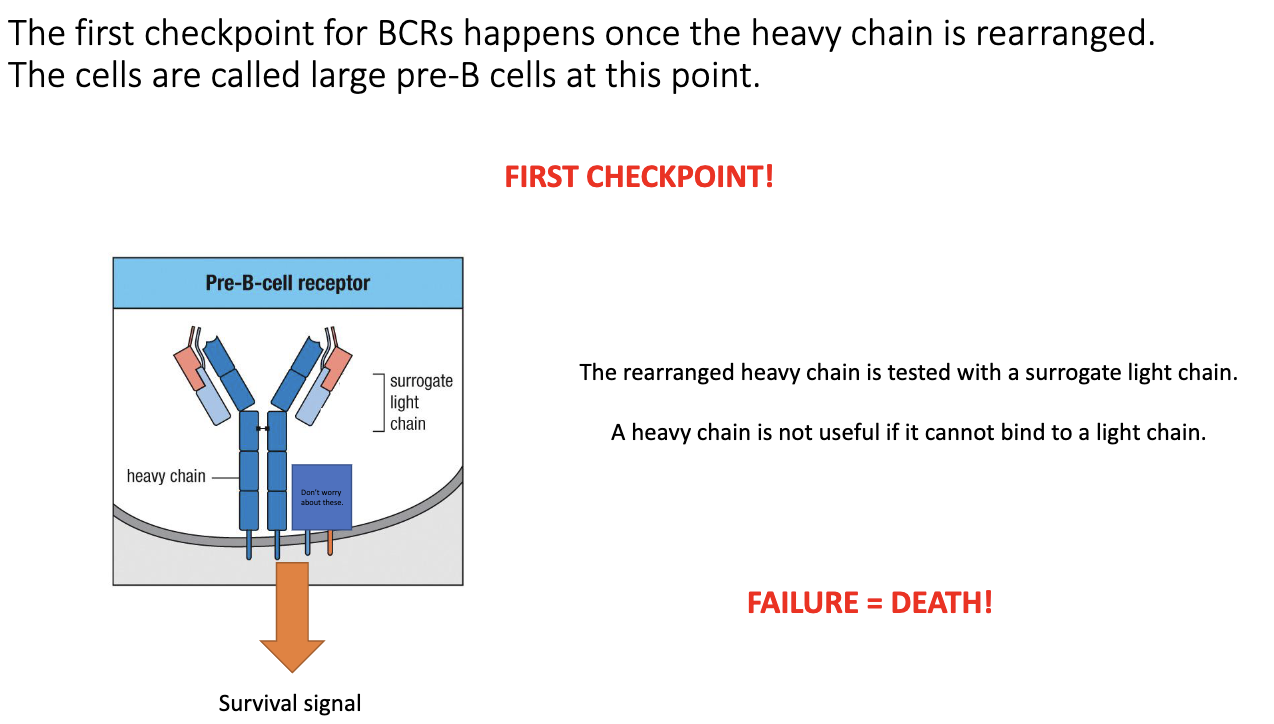
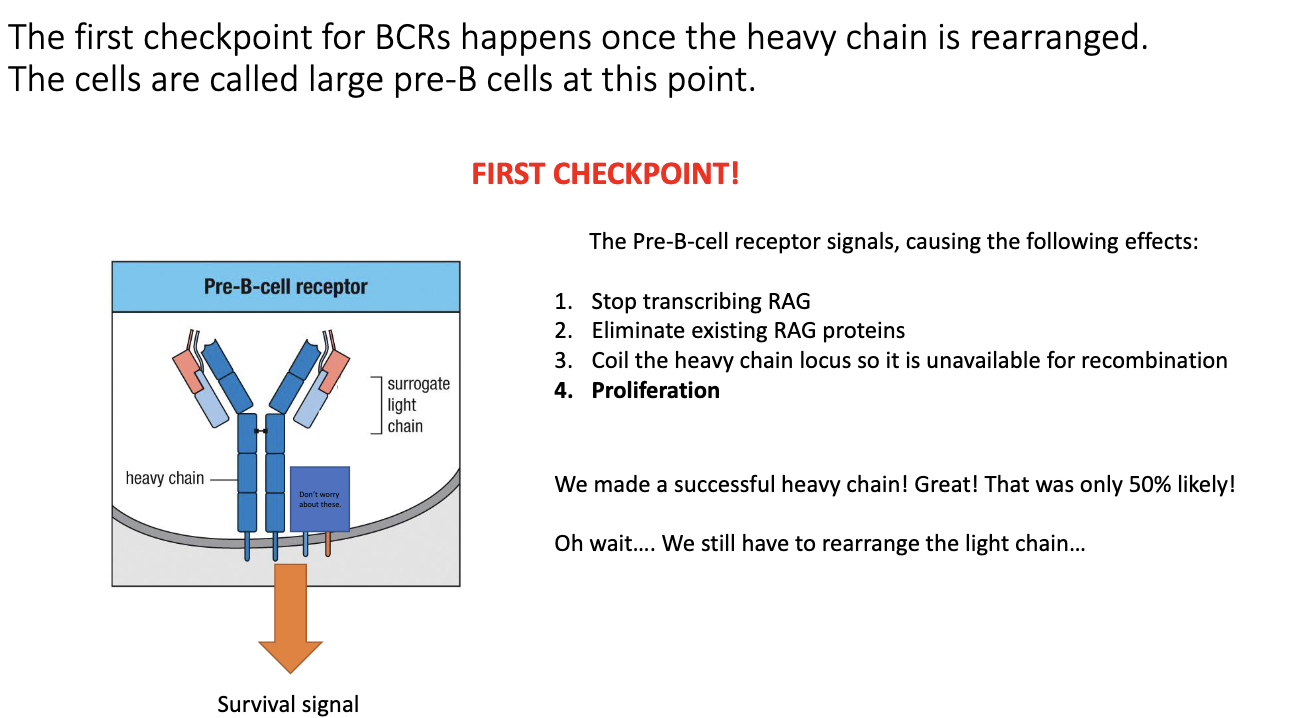
What is the first checkpoint in B-cell development after productive VDJ recombination?
After a productive heavy-chain VDJ, the cell forms a pre-B-cell receptor.
Uses a surrogate light chain (parallel to surrogate α chain in T cells).
If surrogate light chain pairs correctly with the heavy chain → successful signaling → survival.
If the heavy chain cannot pair with a light chain → no signal → death by neglect.
Ensures the heavy chain is both properly folded and able to form a functional BCR.
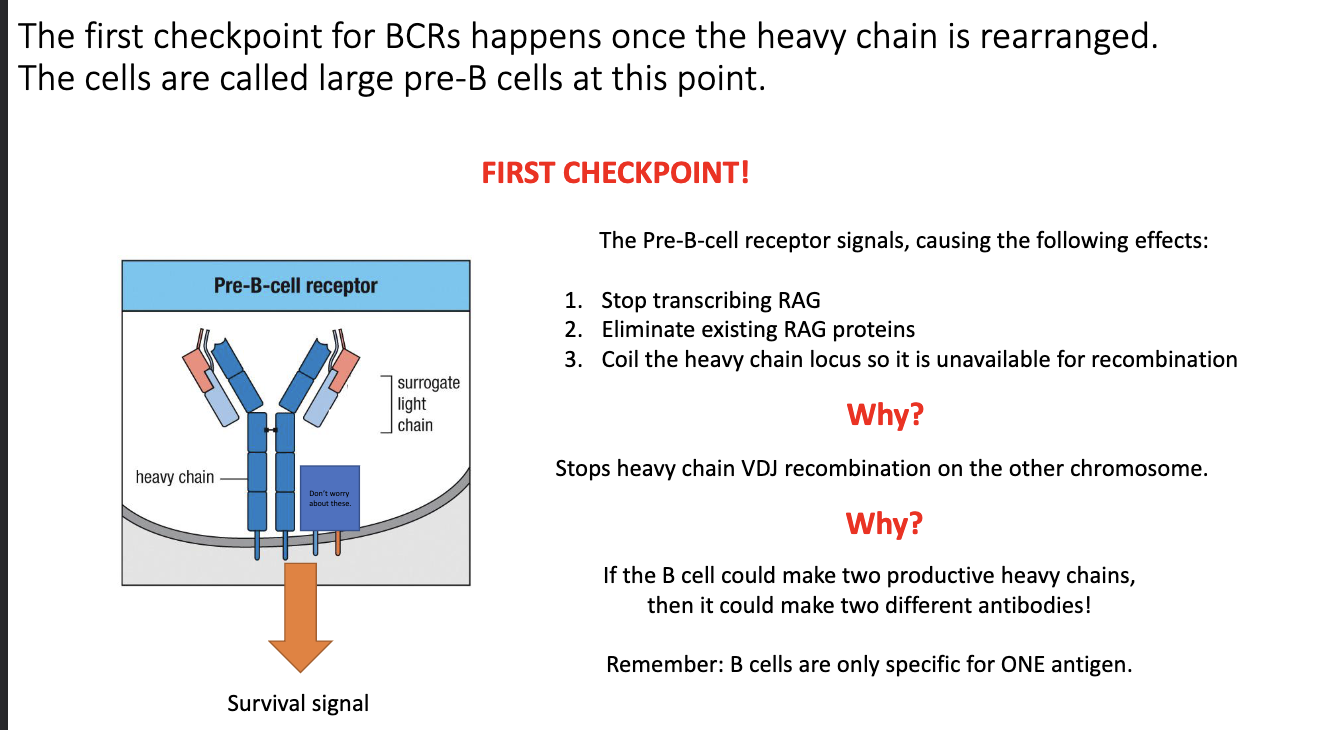
What cellular changes occur when a pre-B-cell receptor signals successfully?
Pre-BCR signaling triggers three events:
Stops RAG transcription.
Destroys existing RAG proteins.
Tightly coils the heavy-chain locus to prevent further rearrangement.
Prevents recombination of the second heavy-chain allele.
Guards against producing two different heavy chains, which would give multiple specificities.
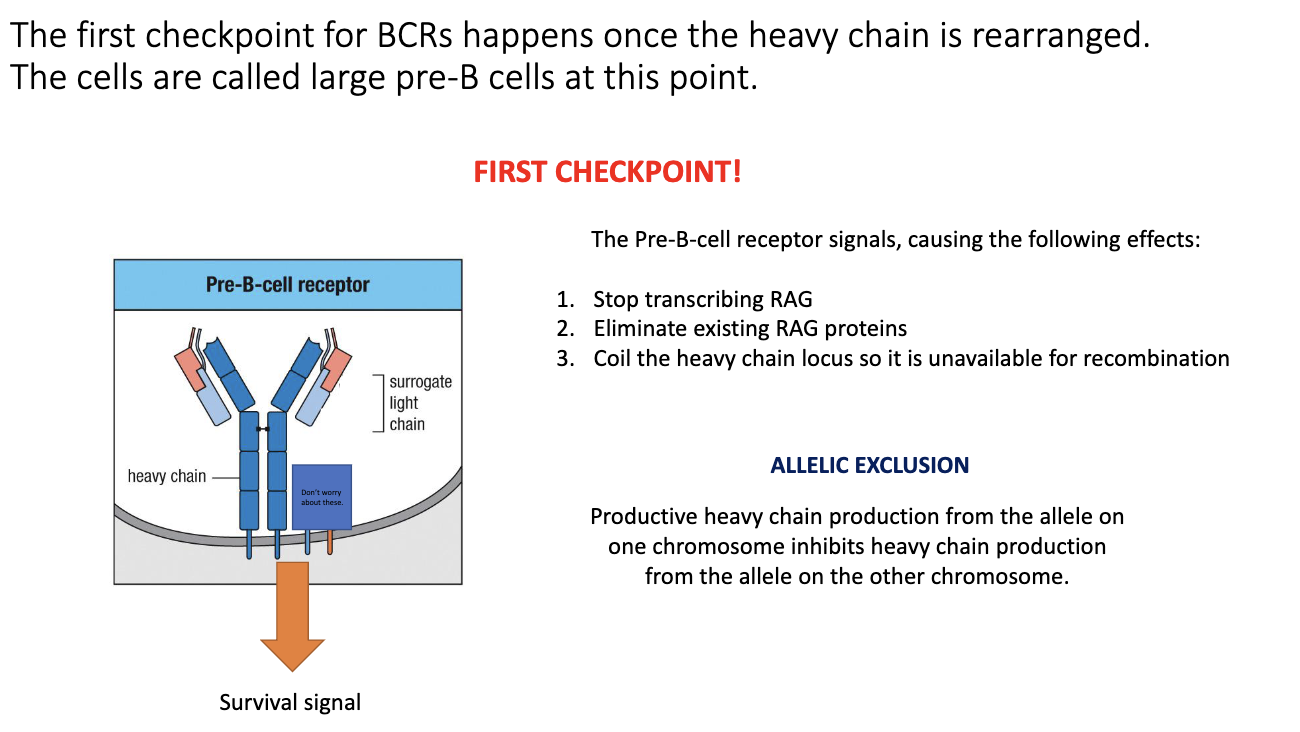
What is allelic exclusion in B cells?
Only one heavy-chain allele completes VDJ recombination.
The other allele stays germline or partially rearranged.
Guarantees each B cell expresses only one antibody specificity.
Essential to avoid mixed or self-reactive specificities.
What happens after a B cell passes the heavy-chain checkpoint?
The cell undergoes proliferation, similar to T cells.
Purpose: expand the small number of cells that successfully made a functional heavy chain.
Produces many daughter cells that will then attempt light-chain rearrangement.
What defines the transition to the large pre-B cell stage?
Everything from stem cell → early pro → late pro leads up to the large pre-B cell.
Called “large” because the cell grows before division; after division, they become smaller.
This stage marks the moment after successful heavy-chain checkpoint.
“μ” heavy chain refers to IgM, meaning the cell has now produced an IgM heavy chain.
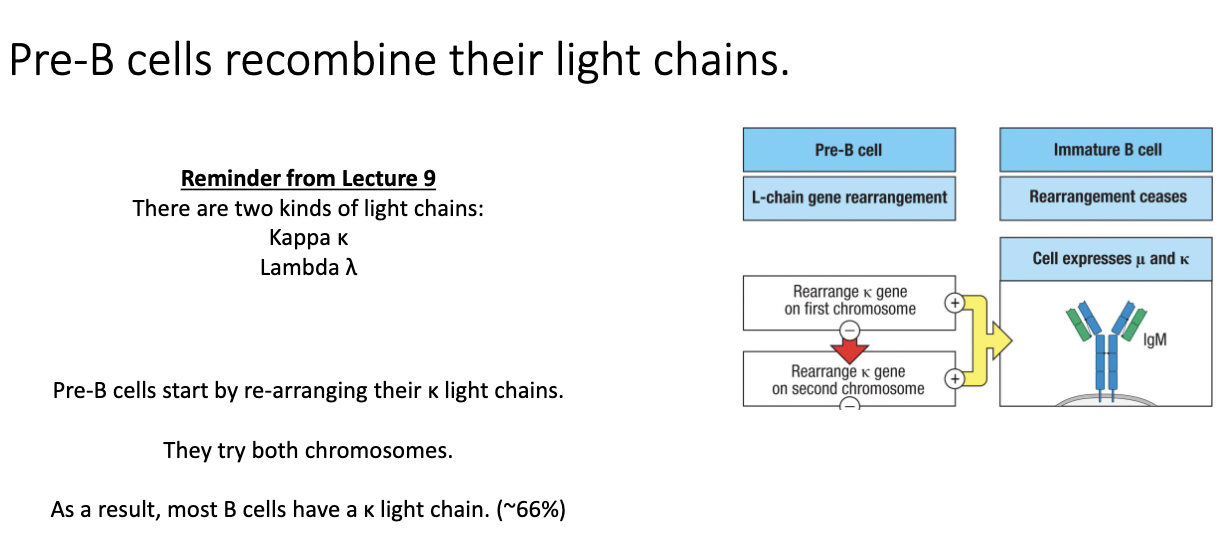
How do small pre-B cells rearrange their light chains, and why is the κ:λ ratio clinically useful?
Small pre-B cells begin light-chain recombination.
Two light-chain types: κ (kappa) and λ (lambda).
Cells always try κ first on both chromosomes.
Most B cells end up expressing κ:
Humans: ~66–75% κ
Mice: ~95% κ
Diagnostic use:
B-cell cancers skew κ:λ ratio.
κ-tumor → very high κ percentage.
λ-tumor → very high λ percentage.
Successful κ rearrangement → IgM heavy chain (μ) + κ light chain → functional antibody.
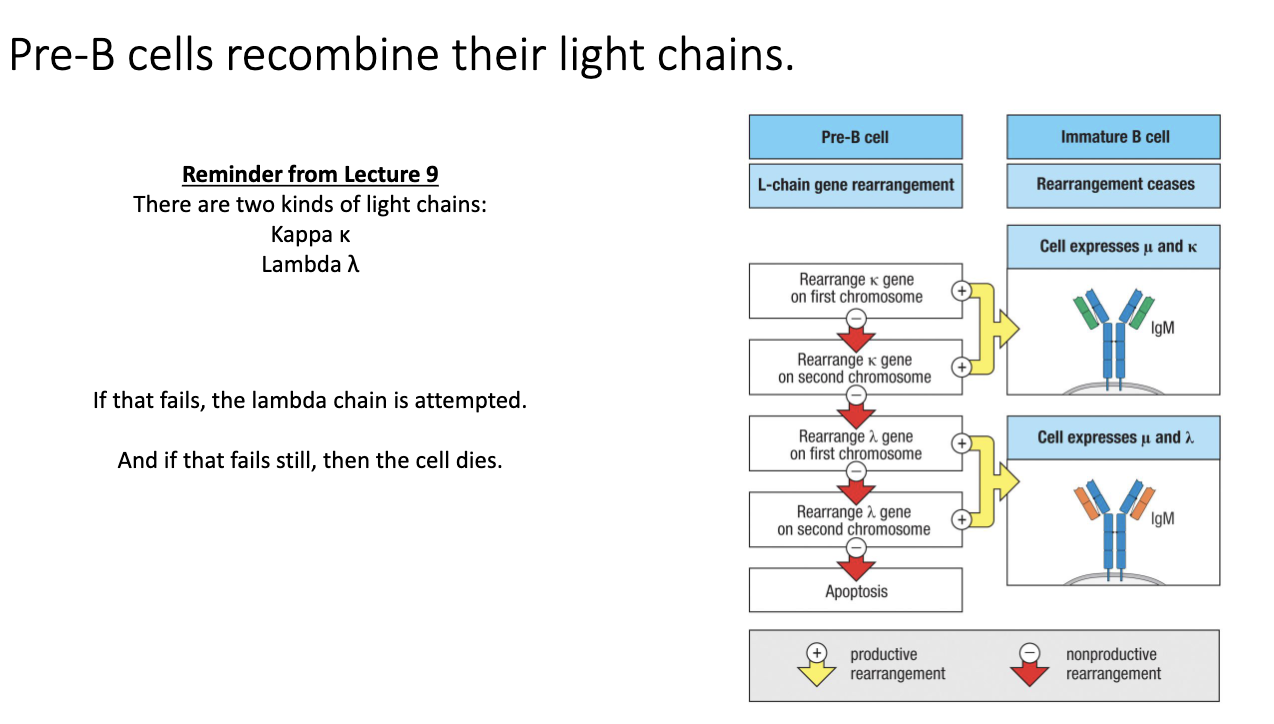
What happens if κ light-chain rearrangement fails?
If κ rearrangement is non-productive, cell switches to λ rearrangement.
Tries λ on both chromosomes.
Successful λ rearrangement → B cell expresses λ light chain.
If both κ and λ fail → apoptosis (cell death).
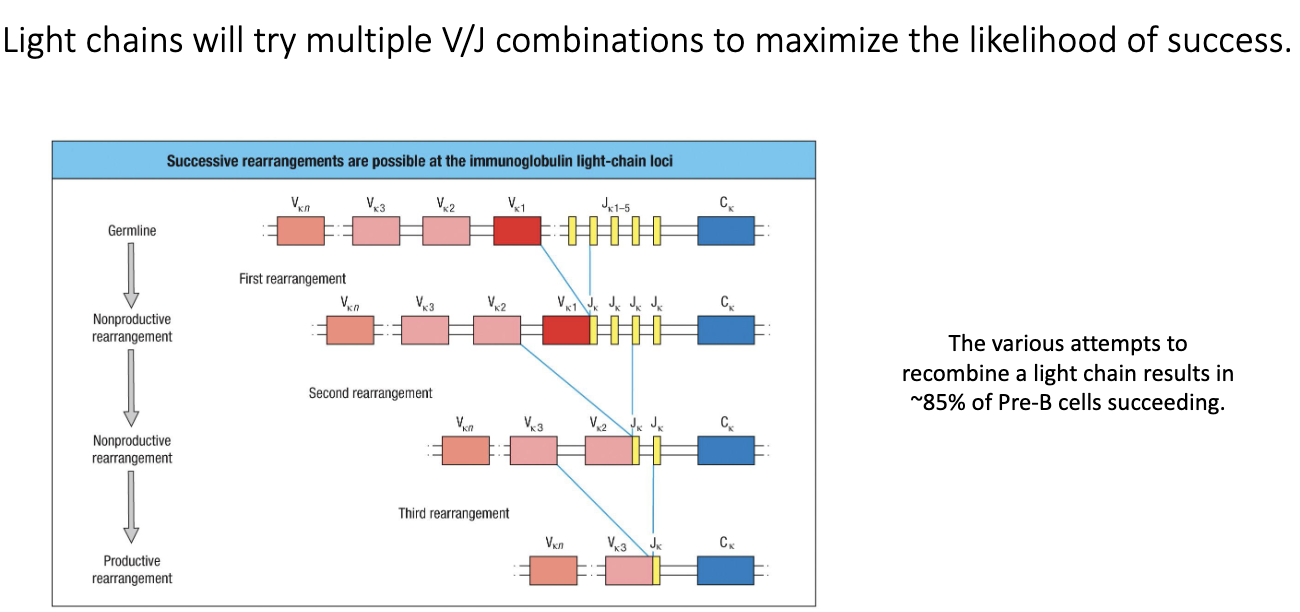
What is unique about repeated rearrangement attempts in light-chain development?
Light chains can repeatedly attempt V–J recombination.
If V–J attempt fails, cell tries another V and another J.
Can retry across both κ alleles and both λ alleles.
This high flexibility lets ~85% of cells successfully form a functional light chain.
Significantly increases BCR diversity and survival.
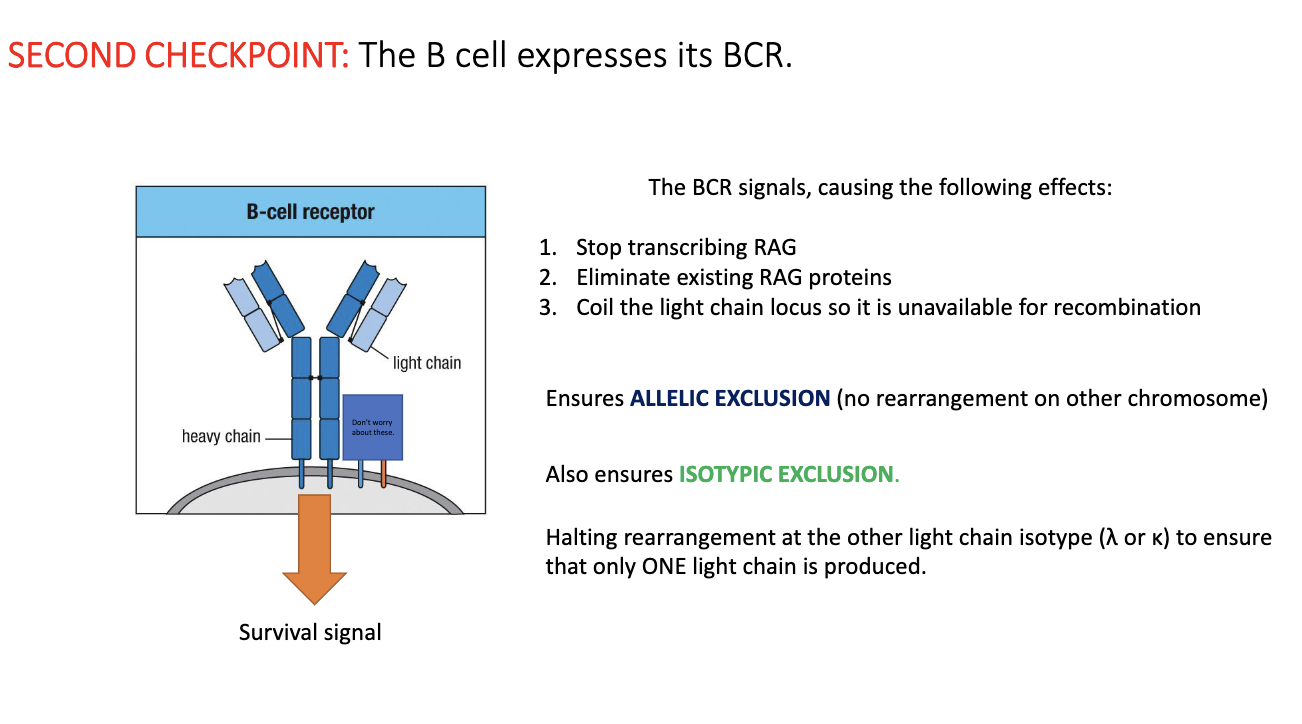
What happens at the second checkpoint of B-cell development?
Cell now has a complete BCR (heavy + light chain).
BCR signaling triggers:
Stop RAG transcription
Destroy RAG proteins
Coil both light-chain loci to block further rearrangement
Ensures allelic exclusion (only one allele used) and isotypic exclusion (only κ or λ, not both).
Result: each B cell expresses one unique BCR.
Why does the immune system enforce allelic and isotypic exclusion for light chains?
Prevents a single B cell from pairing one heavy chain with two different light chains.
Multiple light chains → multiple specificities, which is dangerous.
Antibody specificity is dictated by both heavy and light chain variable regions.
Antibody function (IgM, IgG, IgA, etc.) is determined entirely by the heavy chain.
Light chain exists to expand diversity and ensure proper antibody structure.
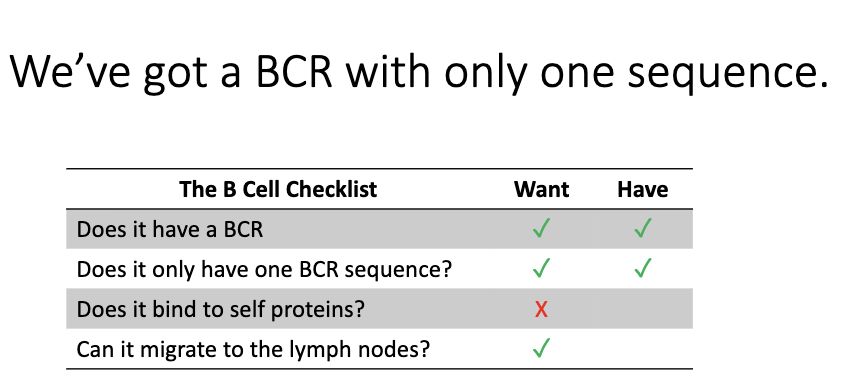
What remains unknown after successfully forming a single B-cell receptor?
At this point, the B cell has:
One BCR sequence
One specificity
But we still don’t know:
Whether the BCR binds self-antigens (must be tested).
Whether the cell can navigate to lymph nodes and function properly.
These requirements must be checked in later developmental stages.
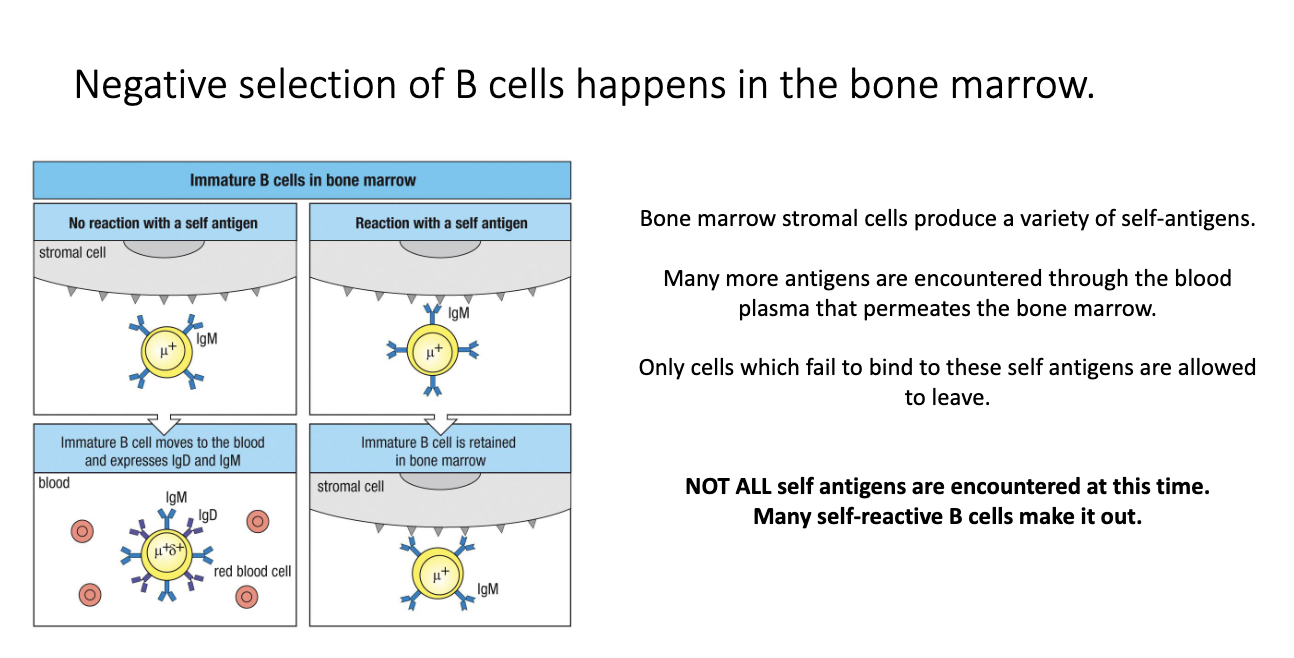
Where does negative selection of B cells occur, and why is it less strict than T-cell negative selection?
B-cell negative selection occurs in the bone marrow.
Bone-marrow stromal cells + blood flow expose B cells to many self-antigens.
Only cells that do NOT bind self strongly can exit to the periphery.
Many self-reactive B cells still escape, because:
T cells are already tightly selected in the thymus → self-reactive B cells usually won’t receive T-cell help, so risk is lower.
Evolution placed far more pressure on controlling self-reactive T cells than B cells.
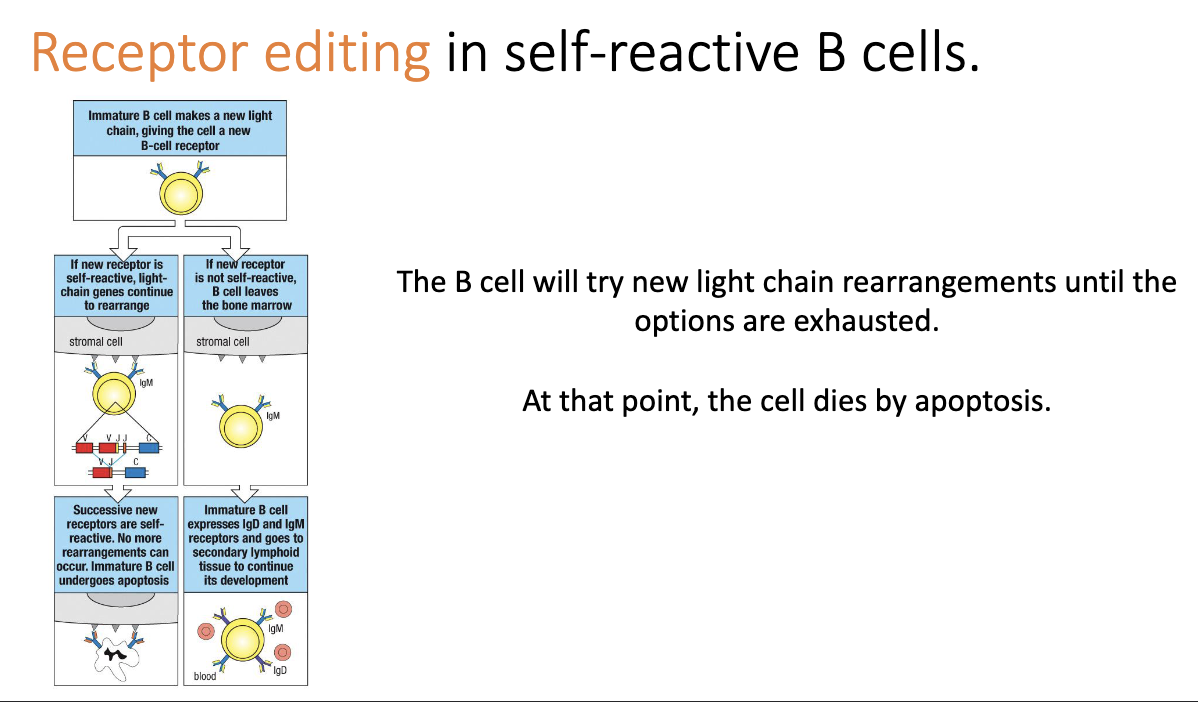
How do B cells attempt to fix self-reactivity during development?
Unlike T cells, B cells can undergo receptor editing.
When a B cell binds self too strongly, it reactivates light-chain rearrangement.
Attempts new V–J combinations to change specificity.
If new light chain removes self-reactivity → cell escapes bone marrow.
Unique to B cells; T cells simply die instead.

What happens if receptor editing fails to eliminate self-reactivity?
B cell keeps trying new light-chain rearrangements until no options remain.
If still self-reactive → apoptosis.
Likely means self-reactivity is driven by the heavy chain, which can’t be edited at this stage.
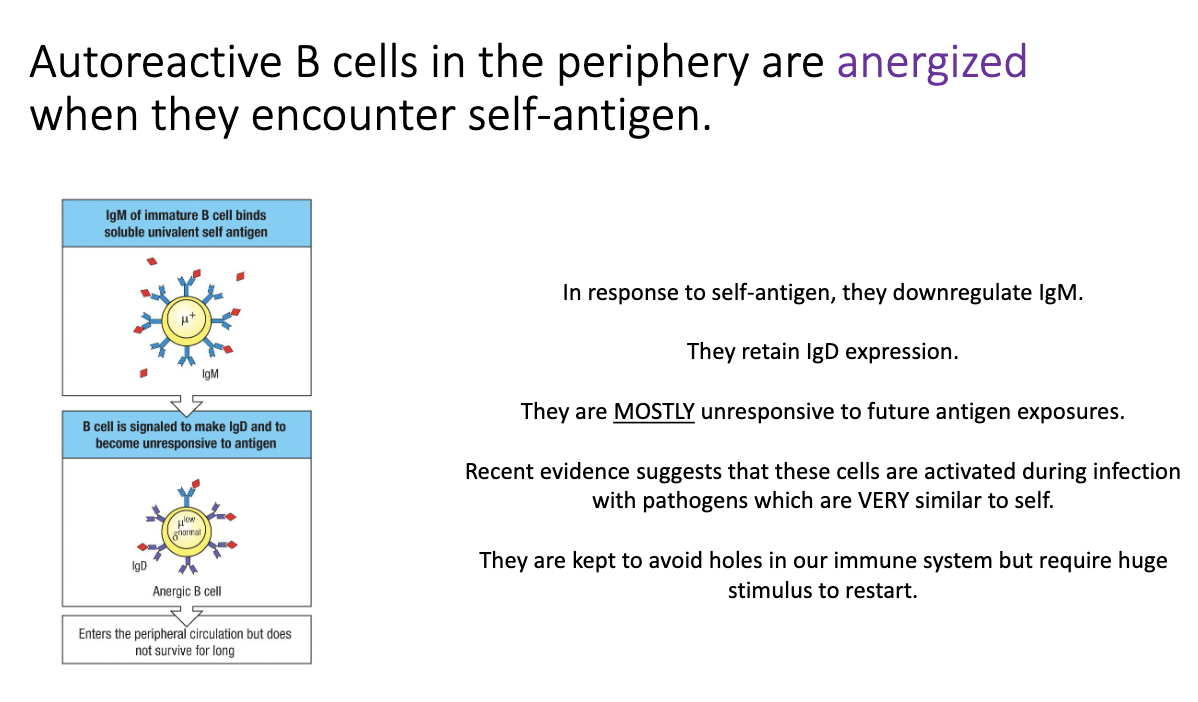
Why are some self-reactive B cells allowed to survive, and what happens to them?
Full deletion of all self-reactive B cells would create holes in immune coverage.
Viruses could exploit this by mimicking self molecules.
Some self-reactive B cells escape but become anergic (functionally silenced).
Anergic B cells:
Turn off IgM, keep only IgD on the surface.
Require a strong antigen signal to wake up.
If a virus mimics self and replicates heavily → enough antigen accumulates → anergic B cell reactivates and adapts specificity to target virus more than self.
What remains after negative selection before a naïve B cell is complete?
After dealing with self-reactivity, the final requirement is migration to lymph nodes.
Only cells that can leave the bone marrow and home to lymphoid tissues become full naïve B cells.
Why do naïve B cells uniquely express both IgM and IgD?
B cells normally express only one heavy-chain isotype.
Exception: naïve B cells express both IgM and IgD.
IgD’s function is unknown:
Humans secrete it, but its role is unclear.
Knockout of IgD → immune response becomes strange, but B cells can still function.
Knockout of IgM → IgD cannot compensate.
Something important about IgD, but mechanism unknown → open research question.

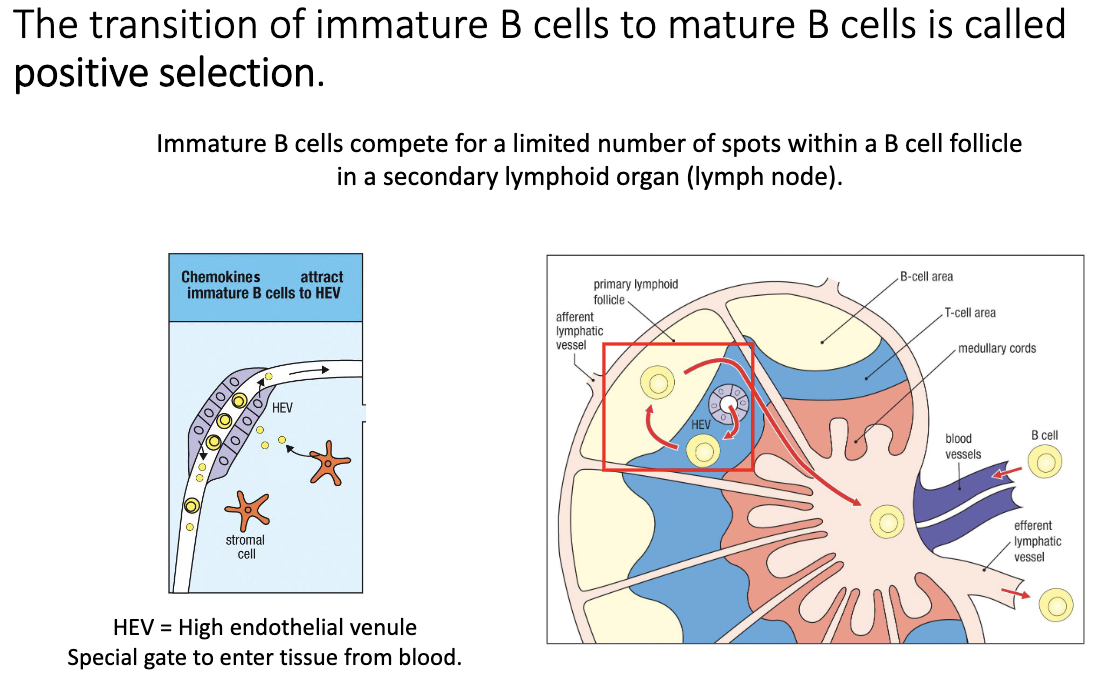
What is positive selection for immature B cells, and where does it occur?
Immature B cells exit bone marrow → enter circulation.
They undergo positive selection after negative selection (opposite order from T cells).
Must successfully enter a B-cell follicle via a high endothelial venule (HEV).
Only cells that can migrate into the follicle survive.
Failure to access follicle → death within a few days.
Success → transition to mature B cells that can survive for weeks–months.
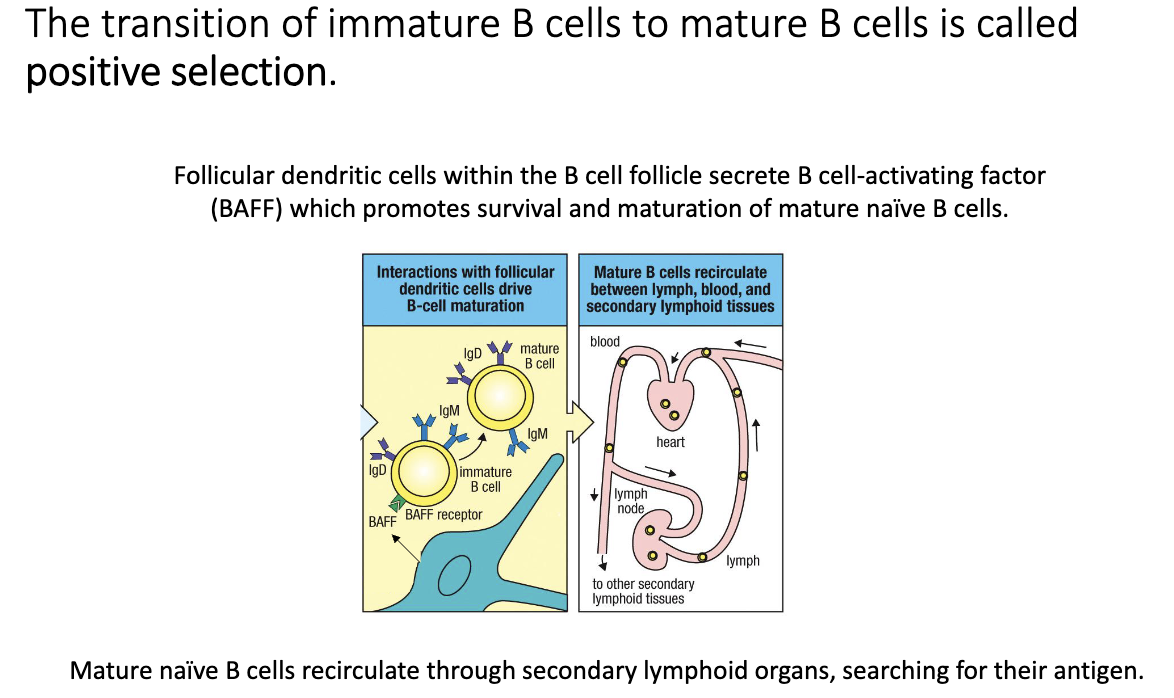
How do follicular dendritic cells support final B-cell maturation?
Follicular dendritic cells (FDCs) secrete BAFF (B-cell activating factor).
B cell must:
Enter follicle via HEV
Bind BAFF
Cells that achieve both (better than competitors) mature into ready-to-activate naïve B cells.
Mature naïve B cells circulate through lymph nodes, spleen, Peyer’s patches, and blood searching for antigen.
What are the survival statistics and lifespan of immature vs. mature B cells?
10–20 million immature B cells exported daily (≈5–10% of total B cell pool).
Most immature B cells die within days.
Mature naïve B cells can live up to ~2 months.
Memory B cells and long-lived plasma cells may persist longer than the lifespan of the organism (decades+).

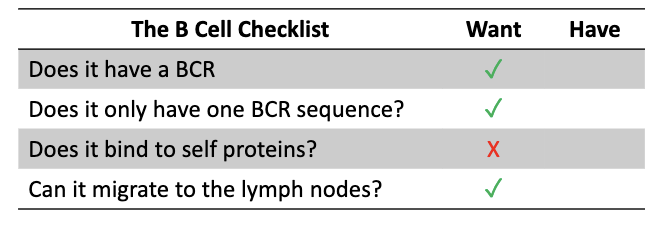
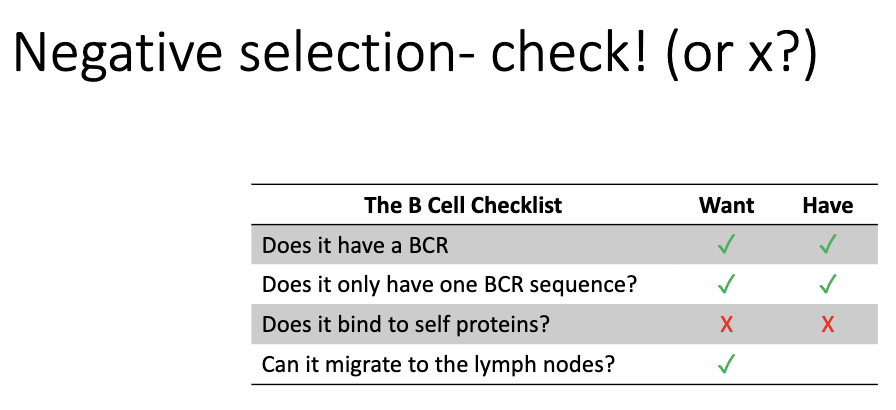
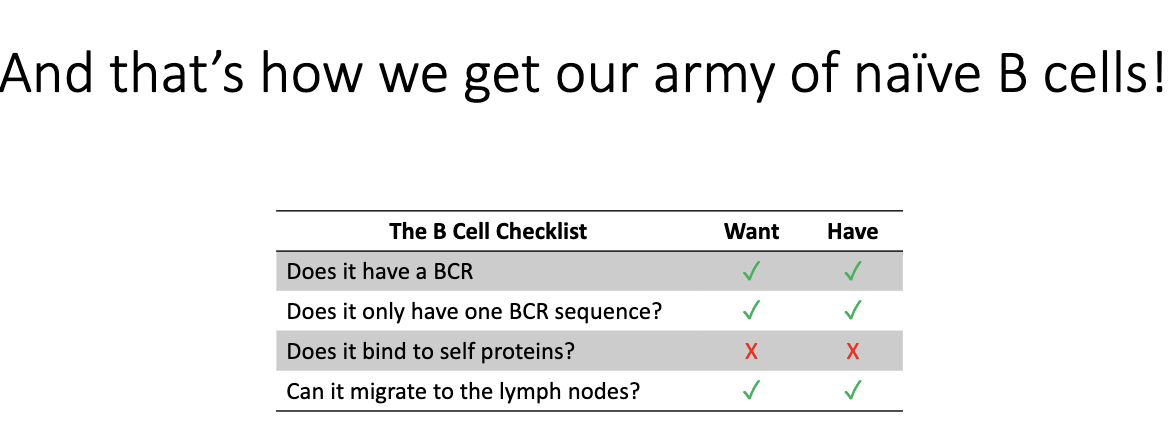
What is the final “B cell checklist”?
BCR ✓
Single BCR sequence ✓
Non-self-reactive X
Can migrate to lymph nodes ✓
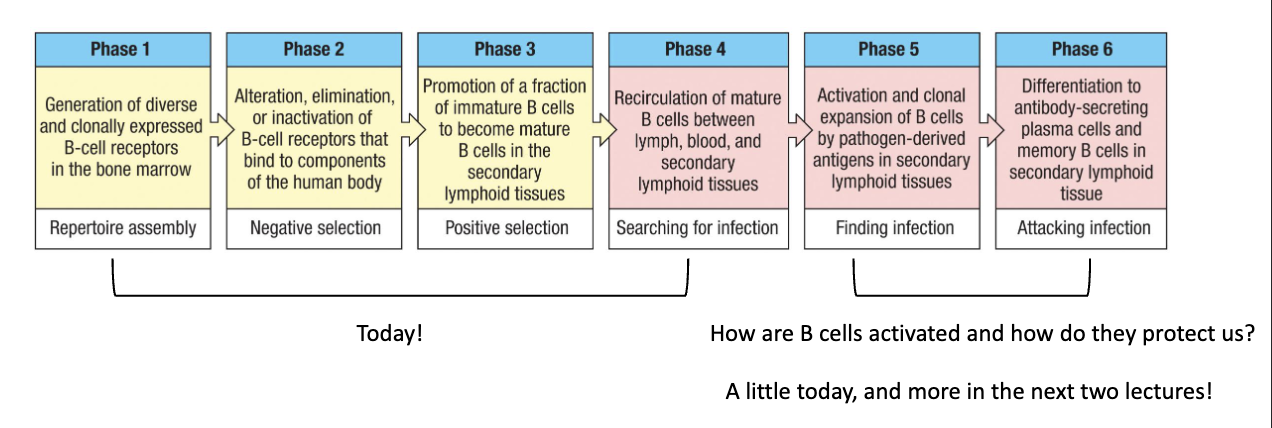
What comes after generating the naïve B-cell pool?
Next topic is B-cell activation:
How B cells find antigen
How they become activated
How activated B cells communicate with T cells
How they initiate an antibody response
What do B cells ultimately do, and what questions remain?
B cells protect via antibodies.
Activation → differentiation into plasma cells → antibody secretion.
Key questions:
How do naïve B cells become activated?
How do they produce antibodies?
How do antibodies neutralize pathogens?
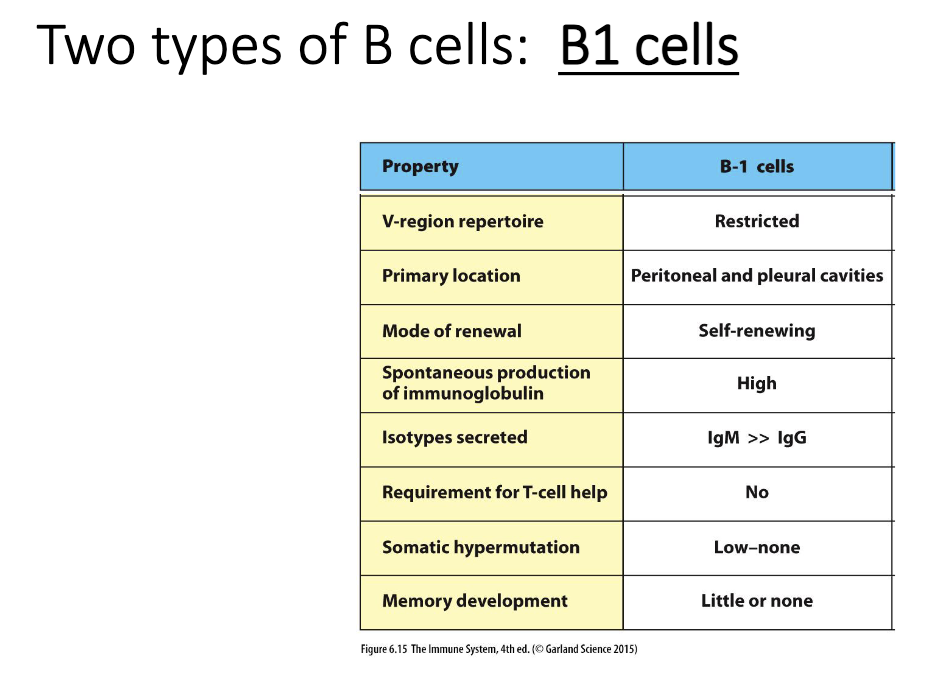
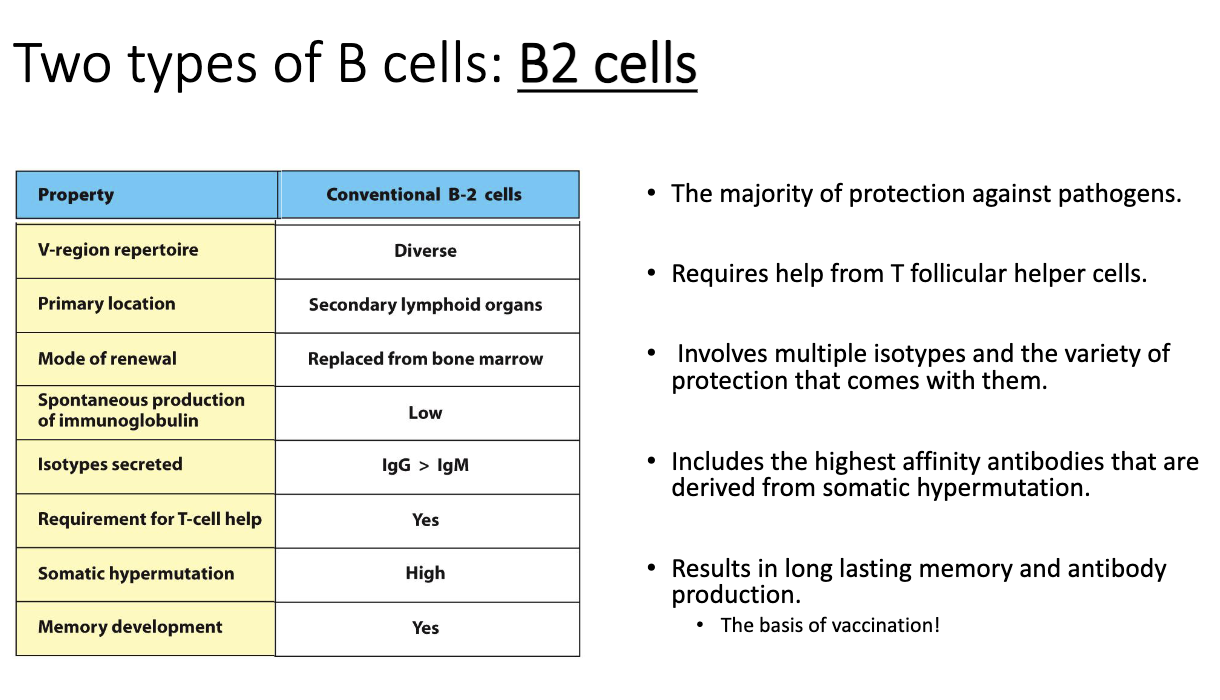
What distinguishes B1 cells from conventional B2 cells?
B1 cells are innate-like, similar to γδ T cells.
Restricted repertoire—recognize limited antigens.
Produce mainly IgM, little switching; minimal somatic hypermutation.
Do not require T-cell help.
Make limited memory.
B2 cells are the conventional, adaptive B cells (not covered here).
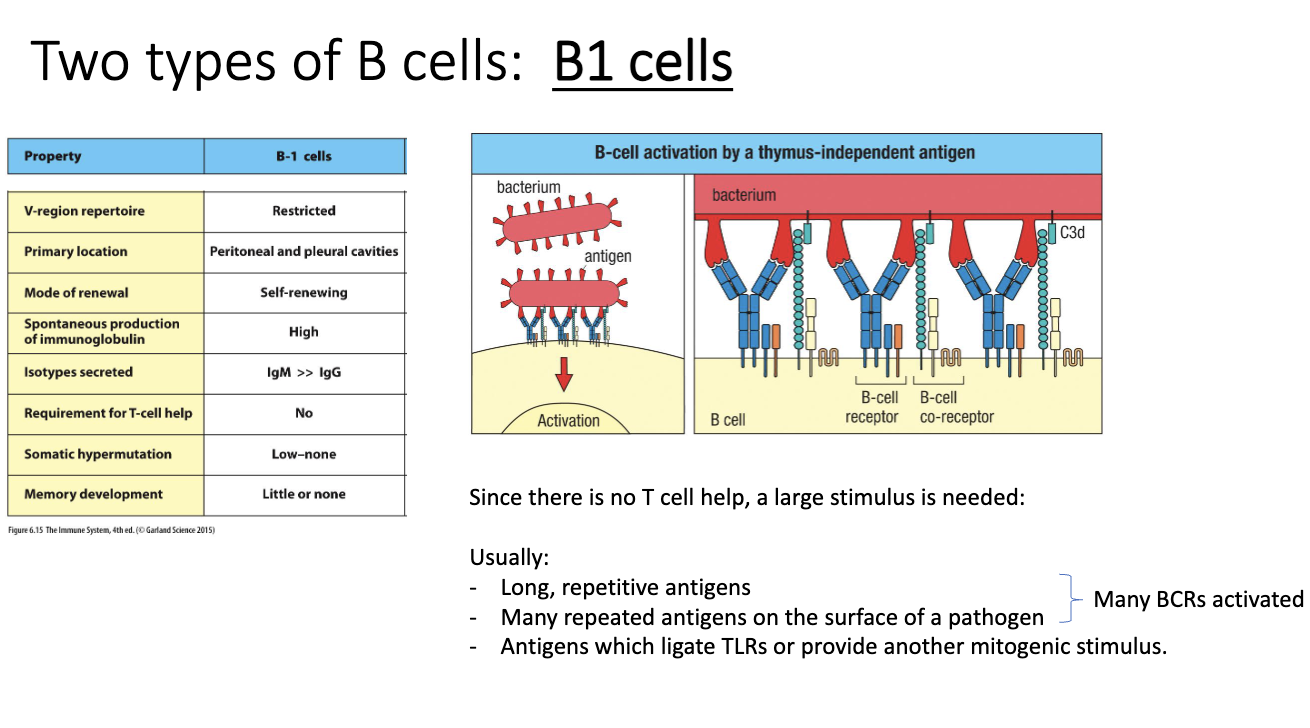
How are B1 cells activated?
B1 cells bind repetitive carbohydrate antigens (common on bacteria).
High repetition clusters large numbers of BCRs → strong activation signal.
Primary location is peritoneal and pleural cavities.
TLRs often provide additional push.
B-cell activation requires crossing a signal threshold:
Many clustered BCRs or
TLR help or
T-cell help (for B2 cells)
Repetitive antigens can activate B1 cells without T-cell help.
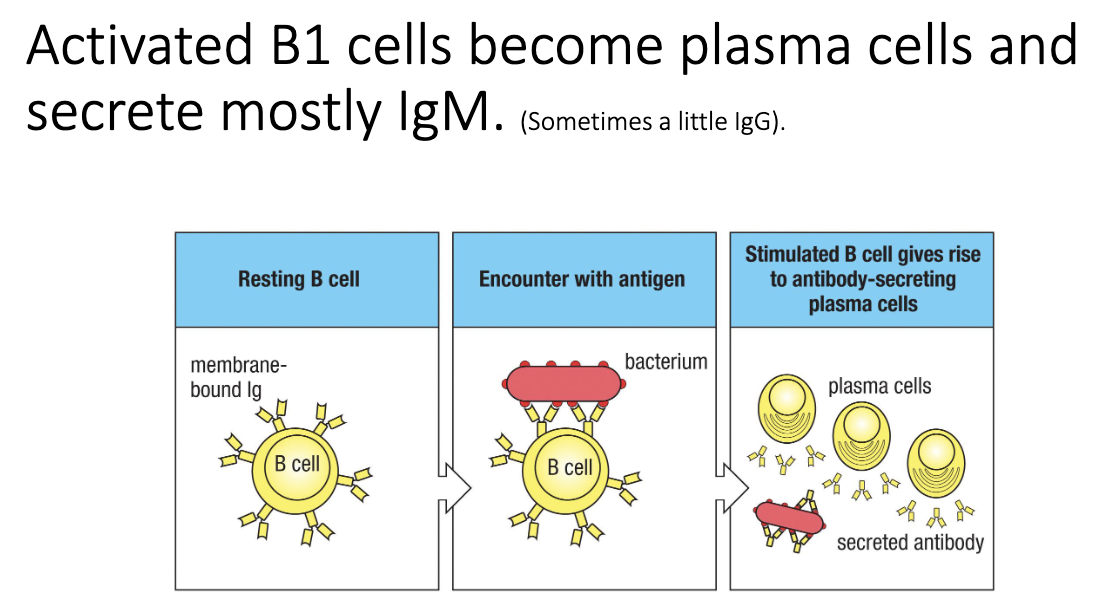
What kind of antibodies do B1 cells make, and how effective are they?
B1 cells mainly produce IgM.
No somatic hypermutation → low-affinity, “crude” antibodies.
Sufficient for early defense:
Bind broadly
Block bacterial attachment
Not high-quality, refined antibodies like those from T cell–dependent responses.
How are B2 cells activated, and what makes their responses so important?
B2 cells mediate most adaptive immunity:
Protective immunity
Autoimmunity
Anti-cancer antibody responses
Allergic responses
Activation is T-cell dependent.
B2 cell process:
B cell binds antigen.
Internalizes & presents it.
T cell confirms antigen match → gives activation signals.
Activated in secondary lymphoid organs (lymph nodes, spleen, Peyer’s patches).
Unlike B1 cells, B2 cells never activate alone—require T-cell help.
T cells provide signals that:
Activate the B cell
Trigger class-switch recombination (IgM → IgG, IgA, IgE, etc.)
Enable somatic hypermutation to improve affinity
Select for improved clones (“affinity maturation”)
Produces memory B cells, essential for long-term protection (vaccines rely on this).
What major B2-cell processes will be covered in following lectures?
How B2 cells become fully activated
How they perform class switching to new isotypes
How affinity maturation improves antibody quality
What antibodies do to protect against infection
Continuation of B-cell biology in later lectures
