Chemistry: Acid and bases strength, conjugate acids and bases
1/35
Earn XP
Description and Tags
My personal study set for CHEM 114 at USD with Dr. Vitt. I recommend shuffle and definition review.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
36 Terms
Strong Acids:
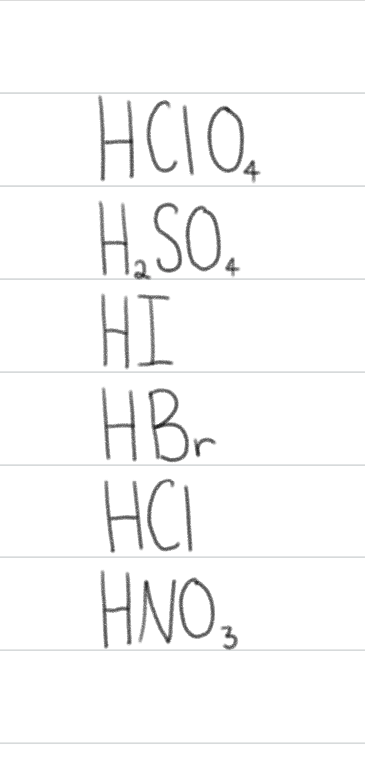
Strong Bases:

pH > 7 means…
the solution is basic
pH < 7 means…
the solution is acidic
pH ~ 7
the solution is neutral

is a ____.
strong acid

is a ____.
strong acid

is a ____.
strong acid

is a ____.
strong acid

is a ____.
strong acid

is a ____.
strong acid

is a ____.
strong base
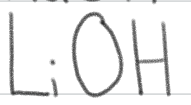
is a ____.
strong base

is a ____.
strong base
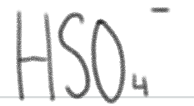
is a _____.
weak acid
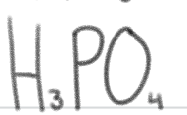
is a ____.
weak acid

is a _____.
weak acid
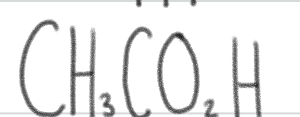
is a _____.
weak acid
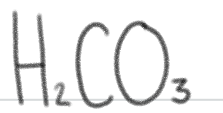
is a _____.
very weak acid
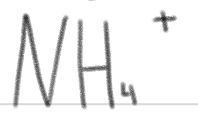
is a ____.
very weak acid

is a _____.
very weak acid
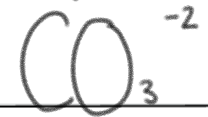
is a _____.
weak base

is a ____.
weak base
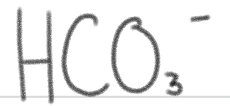
is a _____.
weak base
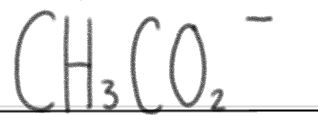
is a _____.
very weak base

is a _____.
very weak base
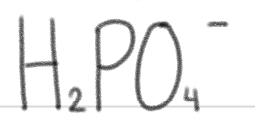
is a _____.
very weak base
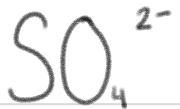
is a ____.
very weak base
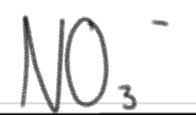
is a _____.
SUPER weak base

is a _____.
SUPER weak base

is a ____.
SUPER weak base
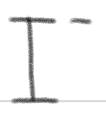
is a ____.
SUPER weak base
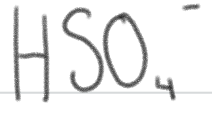
is a _____.
SUPER weak base

is a ____.
SUPER weak base
What does a super weak base mean?
They are incredible weak that they don’t react as bases.
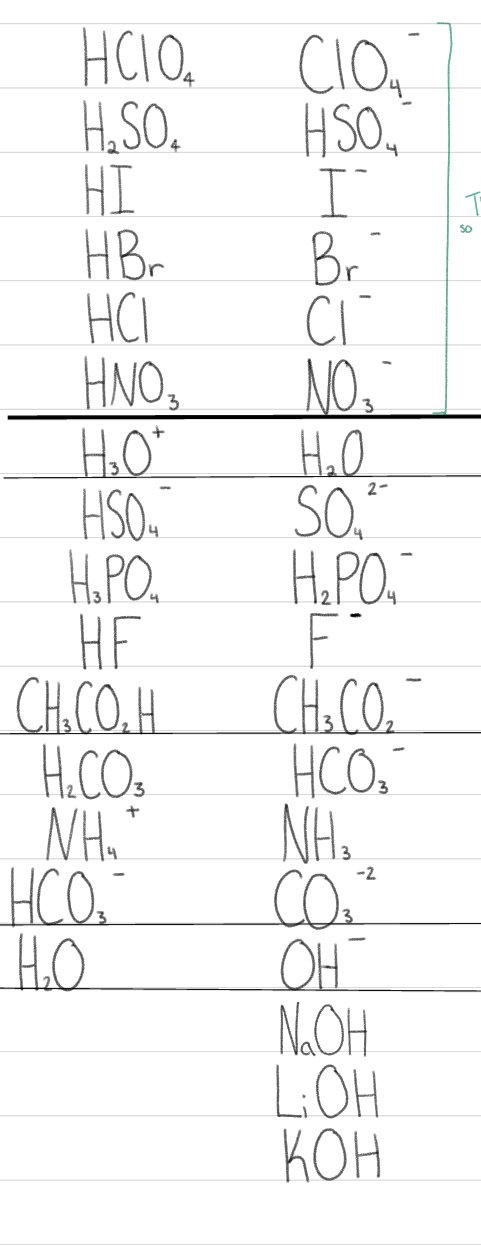
What does the dark black line signify?
everything above it dissociates 100% in H2O