Empirical and Molecular Formula
1/4
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
5 Terms
What is the empirical formula?
Empirical formula is the simplest whole number ratio of atoms of each element in a compound. It is found using molar ratios of each element.
What is the molecular formula?
The molecular formula gives the actual number of atoms of each element in a compound.
How is the molecular formula calculated?
The empirical formula is multiplied by a whole number in order to give the molecular formula.
The relative formula mass (Mr) is required in order to determine the molecular formula when the empirical formula is known.
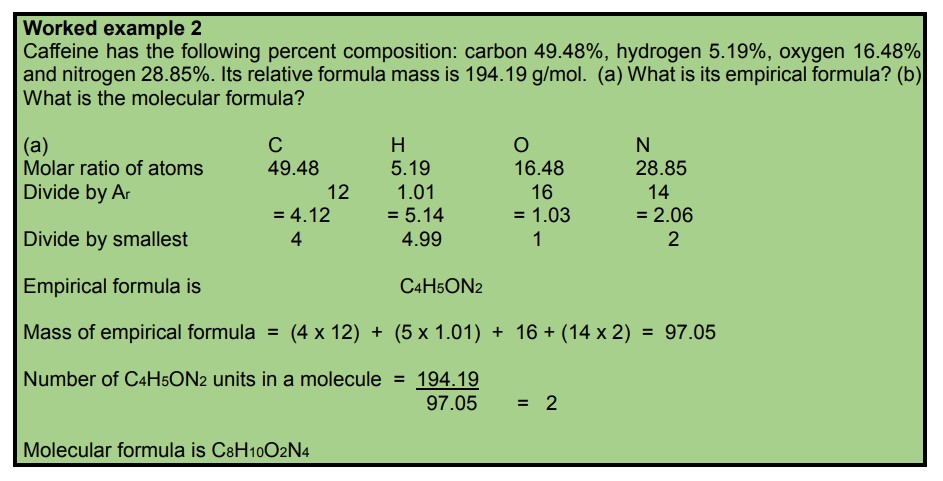
Worked example 1: A compound is found to contain 50.05 % sulfur and 49.95 % oxygen by mass.
a) What is the empirical formula for this compound?
b) The relative formula mass for this compound is 64.0 g mol-1.
What is its molecular formula?
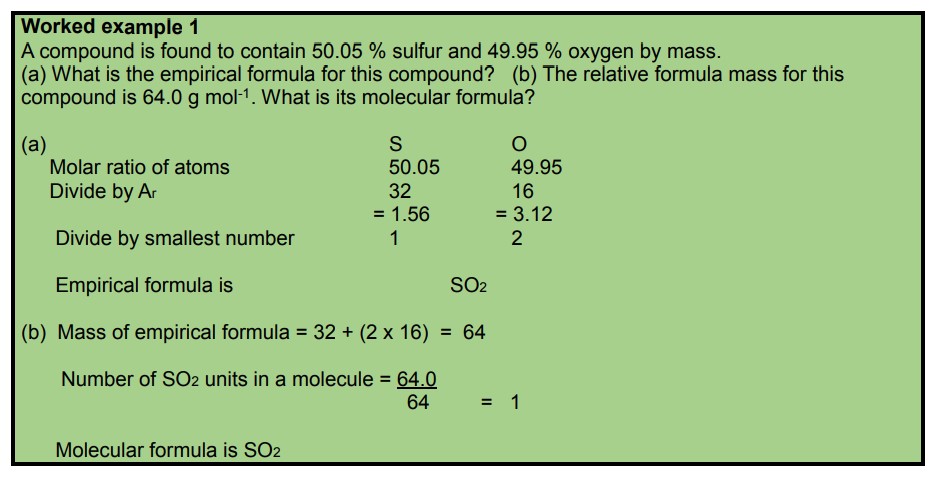
Worked example 2: Caffeine has the following percent composition: carbon 49.48%, hydrogen 5.19%, oxygen 16.48% and nitrogen 28.85%. Its relative formula mass is 194.19 g/mol.
(a) What is its empirical formula?
(b) What is the molecular formula?