Aluminium alloys
1/10
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
11 Terms
Aluminium - Introduction
Used for heat exchangers as very good thermal and electrical conductivity.
Main source of Al is the bauxite ore. Ore is refined to obtain alumina and smelted by electrolytic dissociation into metallic Al.
FCC structure
Low melting temp.
Non-magnetic, non-sparking, reflective, non toxic
Low yield strength
Alloying can increase YS up to 600 MPa
High ductility, good formability, fabricability and machinability
Good corrosion resistance
Weight and stiffness a third of steel
Excellent thermal conductivity
Low electric resistivity
Limit of Al and its alloys is their low strength
Pure Al
High corrosion resistance as the AlO on top of its surface acts as a barrier to corrosion
Formability
Thermal and electrical conductivity
Very low YS
Light
Unsuitable for structural applications
Al benefits a lot from alloying to increase strength by: solid solution strengthening (hinderance to dislocation motion), precipitation hardening, limited strength by second phase hardening, for wrought only: work hardening (more responsive)
Low Yield strength:
Super purity Al is ~10MPa, Commercial purity annealed Al is ~30MPa and ~165MPa when fully cold worked. High performance Al alloys can exceed yield strengths of 550 MPa!
Stiffness about 70 GPa
Duralumin - first age hardened alloy (3.5-4.5% Cu, 0.4-0.8 % Mg, 0.4-1% Mn) which more than doubled the strength.
Al alloys
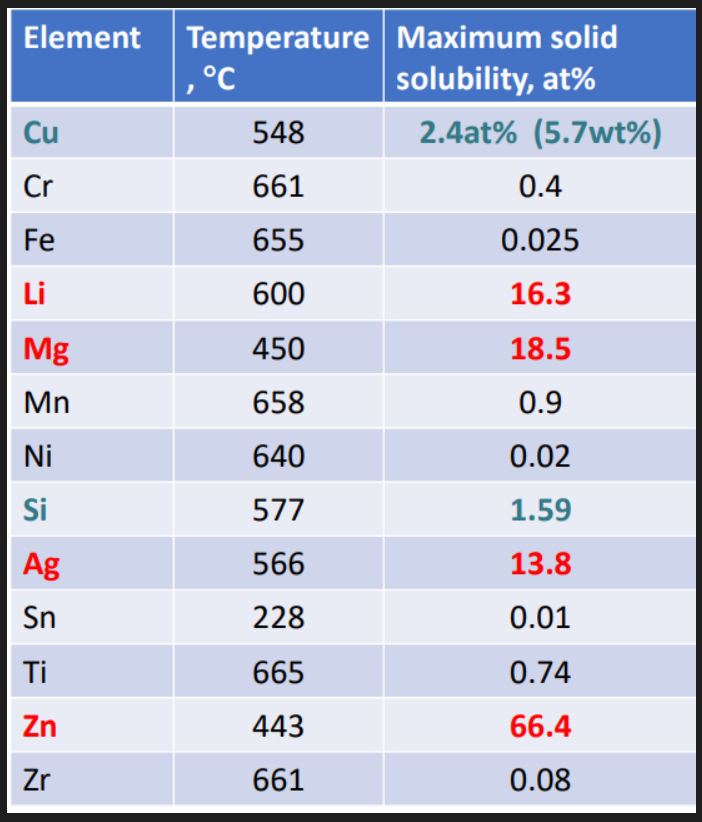
Alloying elements: Cu, Mn, Si, Mg, Zn. These elements all have significant solid solubility in Al. Their solubility increases with increasing temp. These alloys are used to provide increased strength when strain hardened and/or heat treatment.
GP zones are extremely fine-scaled solute enriched regions of the material. Provide hinderance to motion of dislocations.
After quenching, it is a saturated solid solution. Cooled fast to not allow the equilibrium phase to fail.
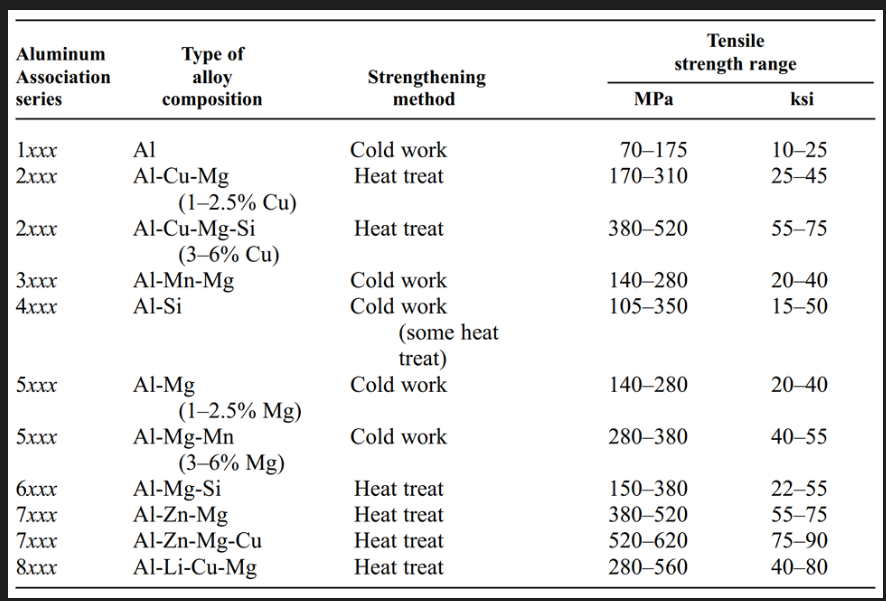
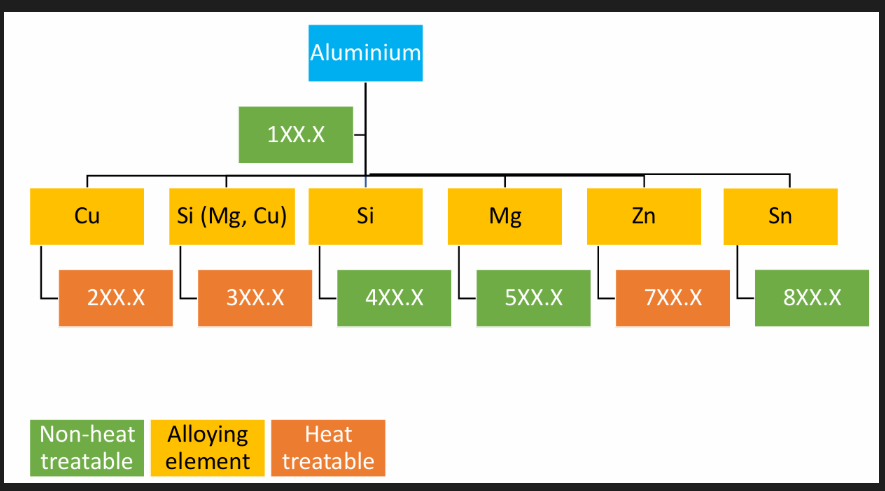
Al alloys classification
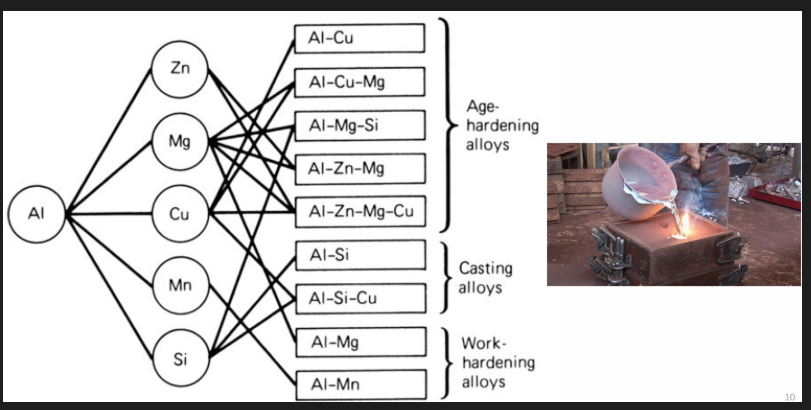
2 main categories:
wrought (hammered) alloys
cast (liquid poured into a mould to get solid shape)
Both of these are then divided into whether they are heat treated or not:
Heat treatable alloys, responsive to precipitation hardening. Heat treatment consists of solution heat treatment, quenching and precipitation hardening.
Non-heat treatable/work hardening alloy: wrought alloys which rely on work hardening through mechanical reduction used in combination with annealing procedures.
Some casting alloys are essentially not heat treatable and are used only in as-cast or in thermally modified conditions unrelated to solution or precipitation effects.
Designation system
When Mg and Si are added together, becomes precipitation hardenable.
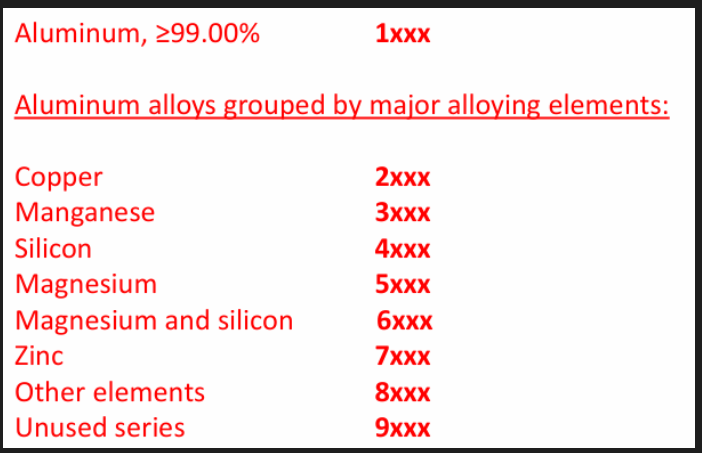
Wrought: 4 digits
First digit indicates the group
For 1xxx, 10xx is used for unalloyed composition. Last 2 digits indicate min Al%
For 2xxx to 8xxx, second digit indicates modification. 0 indicates original alloy. Last 2 digits have no significance but serves to identify alloys. 2, 6 and 7 series alloys are heat treatable.
see image red for types
Cast: 4 digit with decimal point
First digit indicates the alloy group
2xx.x - 8xx.x alloys, second 2 digits identify the specific Al alloy. Last digit indicates product form (0 - casting, 1 - ingot, not cast)
A serial letter preceding the numerical designation ex A indicates modification to original alloys or of impurity limits for unalloyed Al.
see image blue for types

Principal Al alloys
Main strengthening mechanisms in Al alloys
Solute hardening/solid solution strengthening
Crystal lattice distorts (increase with increased difference in atomic radii)
Increase stress required for dislocation movement.
Depends on type and amount of the alloying element. Can increase YS 6 fold
Mn and Mg are examples of elements used with Aluminium.
For solid solution hardening to be possible, the solute must have:
Appreciable solid solubility at the annealing temperature
Able to remain in solution after a slow cool
Not react with other elements to form insoluble phases
Strengthening effect increases with increasing difference in the atomic radii between the solute and solvent.
Zn, Mg, Cu and Si have significant solid solubilities. More than 1%.
Solute hardening strengthens a metal by adding alloying (solute) atoms that dissolve in the base metal to form a solid solution. Solute atoms differ in size or elastic properties from host atoms. This creates lattice distortion. Distorted lattice impedes dislocation motion. More resistance to dislocations → higher strength. Can be substitutional or interstitial. Strength increases without a separate phase. Exs: include brass and steel
Strength ↑, Ductility slightly ↓
Strain hardening by cold working
Applicable for wrought alloys.
Strength increases due to increase in no of dislocations.
Some alloys ex Al-Mg (5xxx) alloys respond very well to strain hardening.
Strain hardening occurs when a metal is plastically deformed below its recrystallisation temperature.
After a metal is cold worked, its grains become stretched and full of defects.
When the metal is heated to the recrystallisation temperature, new, fresh grains grow, restoring ductility and removing work hardening.
Plastic deformation increases dislocation density. Dislocations entangle and block each other. Further dislocation motion becomes difficult. Strength and hardness ↑, Ductility ↓, Yield stress ↑
Examples: Cold rolled sheets, Wire drawing
Precipitation/age hardening
Precipitates as result of a series of heat treating processes (Solution heat treatment then quenching followed by aging) and produce the strongest alloys.
Can increase YS of aluminium up to 15 fold depending on alloy and treatment.
Only certain alloys can be precipitation strengthened (2xxx, 6xxx, 7xxx; 2xx.x,3xx.x,7xx.x)
The heat treatment involves:
Solution heat treatment at a high temperature to maximise solubility (above equilibrium solvus). Solution heat treatments designed to maximise solubility of elements that participate in subsequent aging treatments. They are most effective near the solidus or eutectic temperature (maximum solubility and rapid diffusion rate).
Rapid cooling or quenching to a low temperature to obtain a super saturated solid solution with solute elements and vacancies.
Heat treatment (ageing/precipitation hardening) may then be applied. (below metastable miscibility gap called Guinier-Preston (GP) zone solvus line)
Precipitation hardening strengthens alloys by forming fine precipitates within the metal through heat treatment.
Process steps:
Solution treatment (heat and dissolve solute)
Quenching (trap solute atoms)
Aging (form fine precipitates)
Key points:
Precipitates block dislocations
Strength increases significantly
Over-aging can reduce strength
Second phase constituents
Intermetallic compounds (ex Al12 (Fe, Mn)3Si; Al20Cu2Mn3, Al12Mg2Cr) and elemental Si formed during solidification contribute to strengthening.
Sometimes intermetallic are made to produce fine incoherent precipitates that prevent recrystallisation (control grain structure) and inhibit grain growth.
This mechanism strengthens metals by introducing a second phase (particles different from the matrix). Second phase particles act as obstacles to dislocation motion
Dislocations either:
Cut through particles (if soft/coherent), or
Bypass them (Orowan looping if hard/non-coherent)
Types:
Precipitates
Dispersoids
Carbides
Key effects:
Strength ↑ significantly
Can improve high-temperature performance
Example:
Precipitation-hardened aluminum alloys
Carbide-strengthened steels
Grain size
Grain size reduction to increase strength
Hall-Petch relationship
Strengthening achieved by reducing grain size.
Grain boundaries block dislocation motion
Smaller grains → more grain boundaries
Dislocations must change direction at each boundary → harder to move
Strength ↑
Toughness ↑ (up to a limit)
Examples: Fine-grained steels, Controlled rolling
Wrought Al alloys
Around 85% of total Al is used in the wrought condition
HW or CW applied (rolling, extrusion, drawing) so as to transform cast ingot to the desired product form
Types of mill products:
flat rolled (plate, sheet, foil)
rods, bar, wires
tubular
shapes
forgings
2 and 7 series are the strongest
Wrought super purity and CP-AL (1xxx series)
Indicates super pure Al and commercial pure Al having up to 1% of impurities
Properties:
Excellent corrosion resistance (resistant to SCC, intergranular corrosion and exfoliation)
High thermal and electrical conductivities
Low mechanical properties
Excellent workability and weldability
Pitting is the principal type of corrosion encountered
Strain hardening may be used to moderately increase strength ex: CP Al YS 30 into 165 MPa
Applications:
Chemical equipment
reflectors
heat exchangers
electrical conductors
capacitors and packaging foil
decorative trim
Ex 1100 (99 min Al, 0.12 Cu) used for food packing trays, chemical processing equipment
EX 1350 (99.50 min Al) used for electrical conductors
Heat treatable wrought Al alloys:
Heat-treatable alloys depend on age hardening to develop an enhanced strength (2xxx, 6xxx, 7xxx)
Divided into 2 groups:
Medium strength and weldable exs: (Al-Mg-Si 6xxx) and Al-Zn-Mg (7xxx)
High strength alloys developed primarily for aircraft construction (have limited weldability) exs: Al-Cu (2xxx), Al-Cu-Mg (2xxx), Al-Zn-Mg-Cu (7xxx)
Al-Cu alloys (2xxx series)
Based on Cu, often Mg as a secondary solution
Solution treatment applied to obtain optimum properties after which properties are comparable to/exceed those of low carbon steel.
Ageing sometimes employed to further increase yield strength (reduction in ductility). Inferior corrosion resistance to other alloys.
Can be subject to intergranular corrosion. Therefore when used as sheets they are usually clad with a high-purity Al or Mg-Si alloy (6xxx) to provide galvanic protection (Alclad). Alclad: consists of a sheet with a clad of a thin layer with 1 series or 6 series.
Usually have limited weldability except alloy 2219 (Al-6.3%Cu-0.3Mn).
Temperature limit of 150 oC.
Applications:
Parts structures requiring high strength to weight ratio. Ex: truck and aircraft wheels, truck suspension parts, aircraft fuselage and wing skins, structural parts
Al-Mg-Si (6xxx series)
Based on Mg and Si (up to 1.5wt% each in the ratio to form Mg2Si i.e. 1.7:1 by weight)
Strengthened during heat treatment (age hardening). Alloy first solution treated at about 530 oC to uniformly distribute Mg and Si. Followed by quenching and ageing ex 165 oC for 10 h.
Precipitation upon age hardening occurs by formation of Guinier-Preston (G-P) zones and a very fine precipitate. Alloys are precipitation hardened.
Mg and Si restrict movement of dislocations increasing strength.
May be formed (e.g. extruded) in solution treated condition (T4) and strengthened after forming to full T6 properties by precipitation heat treatment.
Weaker than 7 but more corrosion resistant, weldable and easy to extrude
Main characteristics:
medium strength
good corrosion resistance
immune to SCC
good weldability
Applications:
Architecture extrusions; pipe railings; heavy duty structures required good corrosion resistance; truck and marine; pipeline; automobile body sheet; high strength electric conductor wire/bus 32 conductor; extrusions and forgings for welded structures.
Al-Zn alloys (7xxx series)
Zn in amounts of 1 to 8% is the major alloying element
When coupled with a smaller percentage of Mg results in heat-treatable alloys of moderate to high strength. Increasing Mg+Zn conc. increases strength but decrease the overall corrosion resistance.
The Cu-free alloys of the series have many desirable characteristics: moderate-to-high strength; excellent toughness; and good workability, formability, good resistance to general corrosion, and weldability.
Addition of Cu to the Al-Zn-Mg system together with small amounts of Cr and Mn results in the highest strength Al based commercially available alloys– lower resistance to general corrosion but benefit SCC resistance.
Control over microstructure, composition and heat treatment are often necessary to maintain adequate resistance to SCC and exfoliation.
Example: controlled by: overaging ex T73
Cooling rate after solution treatment
Maintaining a nonrecrystallized structure through the use of additions such as zirconium
Copper or chromium additions
Adjusting the zinc-magnesium ratio closer to 3:1
7xxx series alloys are used in airframe structures mobile equipment and other highly stressed parts. Higher strength 7xxx alloys exhibit reduced resistance to stress corrosion cracking and are often utilized in a slightly over aged temper (ex T76) to provide better combinations of strength, corrosion resistance, and fracture toughness.
7050 and 7150 for upper wing skin due to good fatigue resistance, fracture toughness and compressive strength.
Non- heat treatable Al alloys
Al-Mn alloys (3xxx series)
Mn as major alloying element
Non-heat treatable, stronger than 1xxx series
A limited % of Mn can be added to Al (1.5wt%)– hence used as a major element in only few alloys.
Often small amount of Mg may be added as well to increase strength further.
Mn present as submicroscopic particles of precipitate, and in larger particles of Al6(Mn,Fe) or Al12(Mn,Fe)3Si phases have similar solution potentials almost the same as the solid-solution matrix- not significant sites for corrosion initiation.
Pitting may be encountered but not the more drastic forms of corrosion (SCC, intergranular)
Properties:
Moderate strength
high ductility
excellent corrosion resistance
good formability and weldability
Applications:
General purpose alloy, beverage cans, cooking equipment, heat exchangers, storage tanks, furniture and roofing, garage doors, and other architectural applications.
e.g. 3003 (Al-1.2Mn- 0.12Cu)– (Annealed 35MPa, 20%El; H18 YS165MPa El 2%) most popular general purpose alloy; fuel tanks, chemical equipment, trim
e.g. 3004 (Al-1.2Mn-1Mg)– (Annealed 70MPa, 20%El, H18 YS 250MPa El 1%) for Al beverage cans; storage tanks; building products
Al-Si alloys (4xxx series)
Major alloying element is Si (up to 12%)
Si is present as second phase constituent particles
Si is cathodic to Aluminium solid solution by several 100mVs– still minimal effect on corrosion resistance
Most alloys are non-heat treatable.
Si lowers melting temperature and weight.
Low melting temperature makes it useful for joining applications where a lower melting point than the base material is needed.
When used in welding HT alloys, it will pick some of the elements that respond to HT to a limited extent.
Applications:
filler wire for welding ex: 4043 (5.2Si) and brazing for structural and automotive applications, architecture with dark gray/charcoal anodic oxide finish (alloys with appreciable amounts of Si).
Alloy 4032 (12.2Si-0.9Cu-1.0Mg-0.9Ni) has a low coefficient of thermal expansion and high wear resistance. Well suited for forged engine pistons. YS (T6 temper) 320MPa and El of 7% (strongest of 4xxx)
Al-Mg alloys (5xxx series)
Mg as major alloying element
Contain between 0.8% to 5% Mg
Affects strength very effectively (solid solution).
Alloy 5005 Al-0.8Mg annealed YS:40 MPa, UTS: 125 MPa
Alloy 5456 (Al-5.1Mg-0.8Mn-0.12Cr) annealed YS: 160 MPa, UTS 310MPa. High Elongation ~ 25%
Work hardening rate increases rapidly with increasing Mg content. Ex 5456 H343: YS 300MPa, UTS 385MPa,El 7%
Adding Mg, a lot of solid solution strengthening
Limited on the amount of cold work and safe operating temperatures permissible for higher-Mg alloys (over ~3.5% for operating temperatures above ~65 °C) to avoid susceptibility to SCC. This occurs due to Mg5Al8 precipitating at grain boundaries and slip bands.
These work hardened alloys may also soften over time (age-softening) due to localised recovery within the deformed grains. (Use H3 tempers)
Main characteristics:
moderate to high strength
good resistance against salt water corrosion
good weldability
Applications:
welded applications ex large fuel/milk/grain transport tanks, pressure vessels. Good corrosion resistance- hulls for small boats; super structures of ocean going vessels; chemical plants; good polishability– automotive trim, architecture components.
Misc alloys (8xxx series)
Alloys with wide range of compositions:
Eg 8001 (Al-1.1Ni-0.6Fe) – used on nuclear energy installations where resistance to corrosive attack by water at high temperature and pressure
E.g. 8011 (Al-0.75Fe-0.7Si) is used for bottle caps due to good deep drawing qualities. Also as electrical conductor
E.g. 8280 based on Al-Sn used as bearing alloy for diesel engine motor cars and trucks.
E.g. 8090 (Al-2.4Li-1.9Cu-0.9Zn-0.12Zr) - heat treatable - based on Li to reduce density; increase stiffness. Replaced medium-to-high strength 2xxx and 7xxx in some aircraft applications.
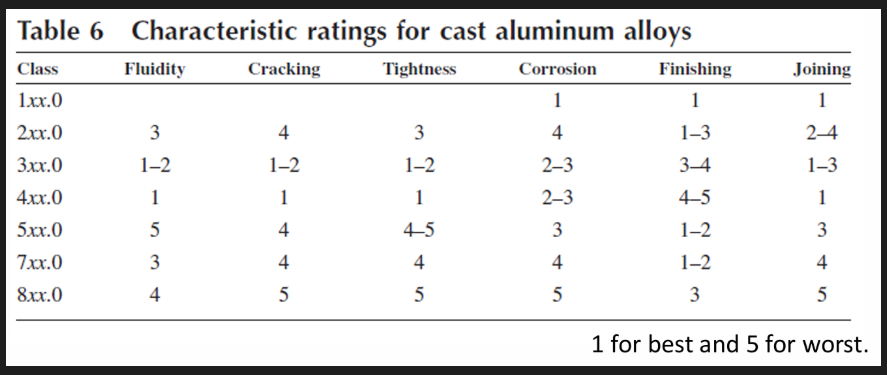
Cast Al alloys
Al is the most versatile of the common foundry metals
Characteristics:
good fluidity for filling thin sections
low melting point
rapid heat transfer to mould (short cycle times) meaning it can solidify quickly
H2 is the only gas with a appreciable solubility in Al but can be readily controlled by processing methods.
Chemically stable
Many of these are free from hot short cracking (tendency of a metal to crack or become brittle at high temperatures, particularly during hot working due to grain-boundary weakening by low-melting impurities) and hot tearing (cracking defect that occurs during solidification, mainly in casting caused by restrained contraction in the mushy state)
Good as cast surface finish with lustrous surface and little to no blemishes
Different from wrought: contain appreciable amounts of Si to be castable
Al-Cu (2xx.0 series)
Capable of developing the highest strengths among all casting alloys- used where strength is a predominant requirement.
These alloys (ex A201.0, 202.0, 204.0, A206.0) contain 4 to 6% Cu and 0.25 to 0.35% Mg, with highly restrictive impurity (iron and silicon) limits, and in some cases also contain 0.25 to 0.35% Mn or Cr and sometimes Ag- fair castability (no second fluid phase, Si fluid phase)
The 2xx.x alloys also have the highest strengths and hardness of all casting alloys at elevated temperatures (to 300 °C). Alloys 222.0, 224.0, 238.0, 240.0, 242.0, and 243.0, some with higher copper contents and up to 2% Mg (6% in alloy 240.0) and additions of manganese, nickel, vanadium, and/or zirconium, are used primarily at elevated temperatures.
Heat treatment is required with the 2xx.x alloys for development of highest strength and ductility and must be properly applied to ensure high resistance to SCC.
General corrosion resistance of these alloys is lower than those of other types of casting alloys, and protection by surface coatings is required in critical applications.
Applications: diesel and aircraft Engine pistons; aircraft cylinder heads; electric hand irons; aircraft wheels
Al-Si (Mg,Cu) (3xx.0 series)
Most popular casting alloys
3xx.x group which in addition to Si (5 to 22%) contain Mg (0.3 to 1%), Cu (0 to 4.5%) or both is the most high-volume used group. (Al-Si-Mg, Al-Si-Cu, Al-Si-Cu-Mg)
Cu, Mg contributes to strength & hardness (as cast F– increased solid solution; artificial aging (T5 or solution + aging (T6, T7)– result of precipitates based on Mg2Si, Al2Cu, Al2CuMg or combinations of). Alloys containing both Cu and Mg have higher strengths at elevated temperatures.
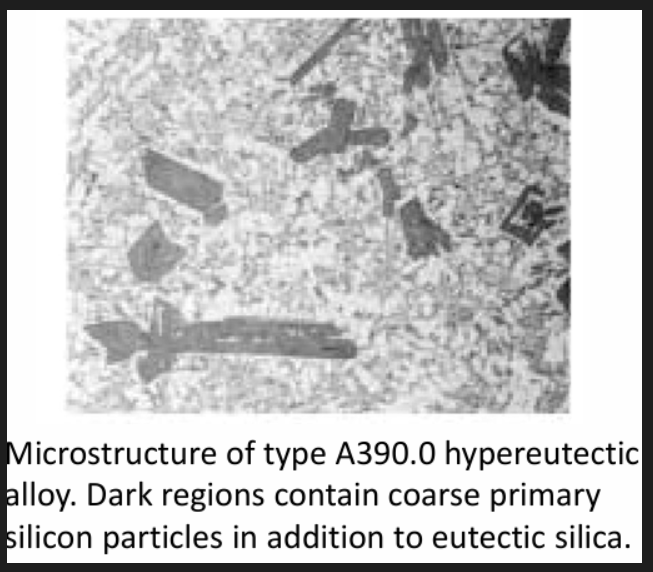
Main role of Si: improves castability and reduces hot shortness. Therefore for more complex castings and permanent mould processes, higher Si alloys are used. Increase Si and Ni products a low benefits applications for pistons and cylinder blocks. If Si exceeds 12% (ex 390.0), primary Si phases are present imparting a good wear resistance.
e.g. 390.0 (17Si-4.5Cu-1.3Fe-0.6Mg) and 380.0 (8Si-2Fe-3.5Cu-0.5Mn-0.1Mg-0.5Ni-3Zn) both used for die casting.
Applications: motor frames and housings (380.0); automotive cylinder block (390.0)
Main role of Mg: form precipitation hardening with Si
Al-Si (4xx.0)
Si is the major alloying element in 4xx.0 alloys which contain from 5 to 12% Si.
These alloys find many applications where combinations of moderate strength and high ductility and impact resistance are required exs: Bridge railing support; utensils, marine fittings 5.2%Si; thin walled intricate, pressure tight castings (12%Si)
The Al-Si eutectic phase impart high fluidity and makes possible the commercial viability of most high volume Aluminium casting. When no copper is added these alloys have good castability and good corrosion resistance.
The microstructure comprise aluminum containing about 1% Si in solid solution as the continuous phase, with particles of essentially pure silicon. Alloys with less than 12% Si are referred to as hypoeutectic, those with close to 12%Si as eutectic, and those with over 12% Si as hypereutectic.
Strength and ductility of these alloys, can be substantially improved by modification of the Al-Si eutectic. Modification of hypoeutectic alloys (<12% Si) is particularly advantageous in sand castings (addition of a controlled amount of Na or Sr or Ca or Sb, which refines the eutectic phase.)
In hypereutectic Al-Si alloys, refinement of the proeutectic silicon phase by P additions is essential for casting and product performance.
Al-Mg (5xx.0)
Single phase binary alloys. Non heat treatable
Moderate-to-high strength and toughness properties.
High corrosion resistance to marine environment; this requires low impurity content by using good handling in the foundry and high quality metals.
Suitable for welding, good machinability, look attractive when anodized.
Relatively poor castability since it does not contain Si. Mg tends to react with oxygen very quickly and therefore melting and pouring practices need to be done very carefully (increasing cost).
Oxide inclusions will also reduce the surface finish for applications where a high polish is needed.
Used for: welded assemblies, architectural and decorative applications; dairy and food handling applications, fitting for sewage use (4%Mg T4YS~90MPa ); marine fittings, ornamental parts (8%Mg); aircraft fittings, railroad/passenger car frames (10%Mg–T4YS- 180MPa)
Al-Zn (7xx.0)
Al-Zn-Mg alloys: undergo natural ageing therefore after casting when left in room temperature conditions, the hardness and strength will increase (20-30 days). This can be accelerated by artificial ageing. The high temp solution treatment and drastic quenching needed for Al-Cu and Al-Si-Mg is not needed for optimum properties in most Al-Zn-Mg alloys.
Characteristics:
Moderate to good tensile properties; good machinability; not recommended for elevated temperature service; good general corrosion resistance; good finishing characteristics, poor castability.
Applications:
Ex alloy 712.0: - 5.8%Zn-0.6Mg -0.5Cr-0.2Ti – (good properties without HT ex large parts) marine castings; machine tool parts - good strength; impact resistance. YS 170 TS 240 El 5% (F or T5 temper)
Al-Sn (8xx.0)
Alloys with ~6%Sn and small amounts of Cu and Ni for strengthening are used for bearing materials (tin imparts lubricity).
Bearing performance is affected by casting method- fine interdendritic distribution of tin, gives optimum bearing properties. Tin forms a very separate phase, a fine tin layer
Used where excellent bearing qualities are required (e.g. oil lubricated heavy duty gas turbines thrust bearings) due to a high load capacity, high temperature capability and good fatigue and corrosion resistance in lubricated environments.
Ex alloy 850.0 (6.3Sn-1Cu-1 Ni) Other applications include connecting rods and crankcase bearings for diesel engines.
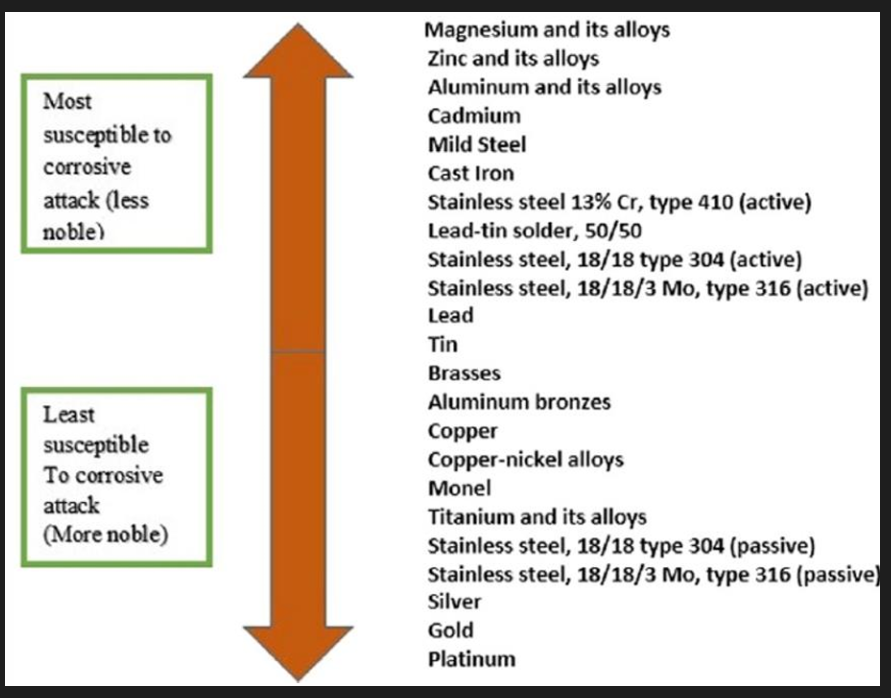
Corrosion
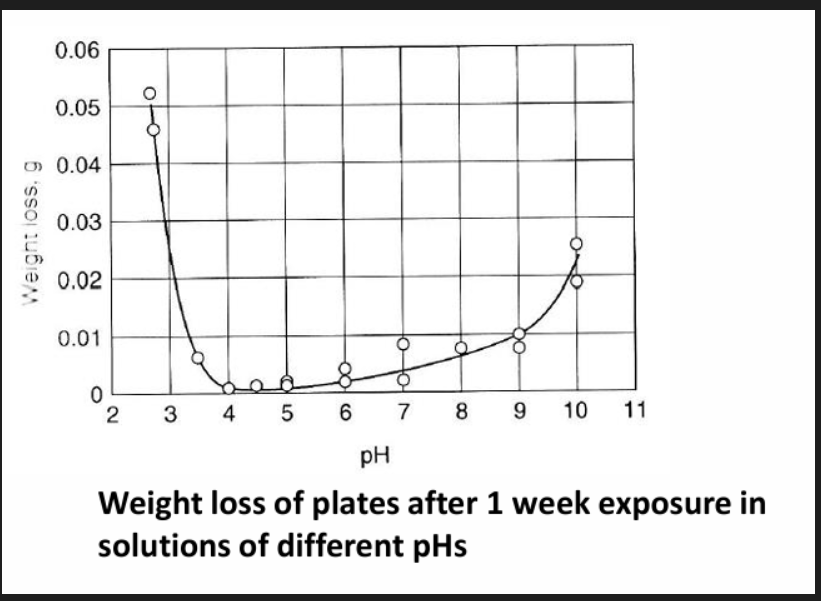
Al is a very active metal but still displays excellent corrosion resistance in most environments due to the rapid formation of a very thin (nanometric) Al oxide. At higher temperatures and humidity the growth rate of the film increases. In water the film contains hydroxide rather than just oxide which makes it less adherent therefore less protective. As a general rule the film is stable at a pH of 4.5 to 8.5 beyond which it will dissolve resulting in rapid corrosion of the base metal.
Al tends to be good at pH from 4 to 8 and 9.
Weight loss - material has been removed from its surface due to some physical or chemical or process. Weight loss is measured to understand how the material is degrading. Main causes are:
- corrosion:
- Chemical reactions with the environment (oxygen, water, acids, salts, etc.) remove metal atoms from the surface.
- Rusting of steel, pitting in aluminum, and tarnishing of copper are examples.
- wear/abrasion:
- Rubbing, grinding, erosion from flowing particles, friction in moving parts.
- Weight loss indicates how resistant the alloy is to wear.
- oxidation: At high temperatures, metals form oxide layers that may flake off, causing weight loss.
- chemical dissolution:
- Acids or other chemicals can dissolve metal surfaces, reducing mass.
What weight loss tells you
- How fast the material is degrading
- How durable the alloy is in its environment
- Whether the alloy is suitable for the intended application
- The corrosion or wear rate, often calculated from the mass lost over time
Tribology
Al-Sn alloys used for tribological applications since 1940 for cast bearing materials.
Wear resistant alloys are based on alloys containing hard, brittle silicon phase. Increasing amount of Si particles generally leads to better (abrasive/sliding) wear resistance.
Additions of Cu, Mg, Fe can further improve the wear resistance by increasing volume fraction of intermetallic silicon bearing phases and (Mg, Cu) impart additional strengthening via age hardening.
Major wear types include: abrasive and sliding wear applications.