Halogen Compounds
1/12
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
13 Terms
Halogenoalkanes
Hydrocarbon with one or more halogen
Production
Free radical substitution
electrophilic addition
Substituiton of alcohols
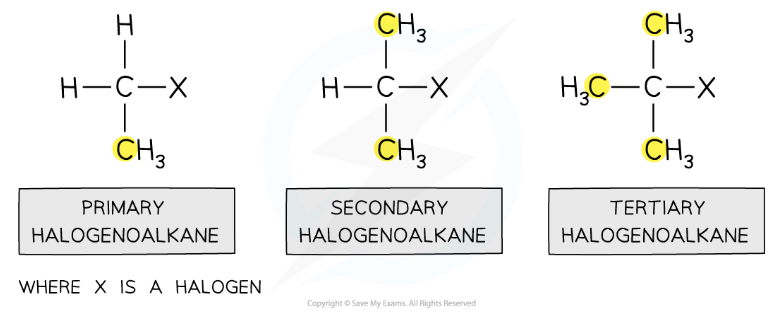
Classifying Halogenoalkanes
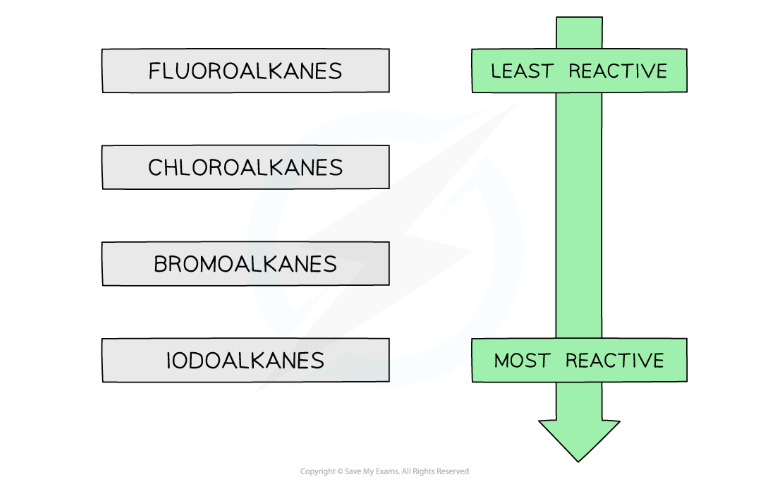
Reactivity of halogenoalkanes compared to alkanes
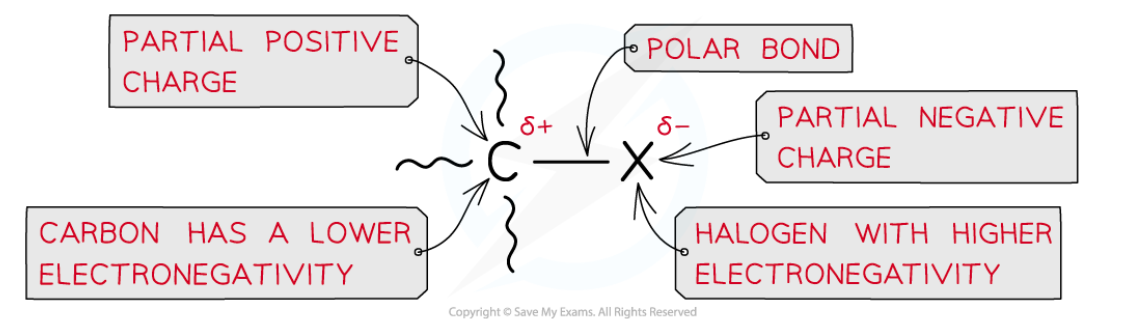
Halogenoalkanes are much more reactive than alkanes due to the presence of the electronegative halogens
The halogen-carbon bond is polar causing the carbon to carry a partial positive and the halogen a partial negative charge
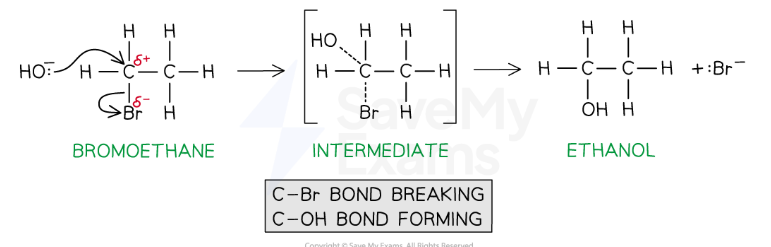
Reaction with NaOH(aq)
The halogen is replaced by the OH-
The aqueous hydroxide (OH- ion) behaves as a nucleophile by donating a pair of electrons to the carbon atom bonded to the halogen
For example, bromoethane reacts with aqueous alkali when heated to form ethanol
Hence, this reaction is a nucleophilic substitution
The halogen is replaced by a nucleophile, :OH–
CH3CH2Br + :OH– → CH3CH2OH + :Br–
Reaction with KCN
nucleophile: CN
Ethanolic solution of potassium cyanide (KCN in ethanol) is heated under reflux with the halogenoalkane
The product is a nitrile
The nucleophilic substitution of halogenoalkanes with KCN adds an extra carbon atom to the carbon chain
Reaction with NH3
Nucleophile: NH3
An ethanolic solution of excess ammonia (NH3 in ethanol) is heated under pressure with the halogenoalkane
It is very important that the ammonia is in excess as the product of the nucleophilic substitution reaction, the ethylamine, can act as a nucleophile and attack another bromoethane to form the secondary amine, diethylamine
Reaction with aqueous silver nitrate
Halogenoalkanes can be broken down under reflux by water to form alcohols
Slower than NaOH as OH- is a stronger nucleophile compared to water
For example, bromoethane reacts with aqueous silver nitrate solution to form ethanol and a Br- ion
The Br- ion will form a cream precipitate with Ag+
Water and Hydroxide

Sn1 and Sn2
These reactions can occur in two different ways (known as SN2 and SN1 reactions) depending on the structure of the halogenoalkane involved
Sn2
Primary and secondry carbocations
One step process
The nucleophile donates a pair of electrons to the δ+ carbon atom to form a new bond
At the same time, the C-X bond is breaking and the halogen (X) takes both electrons in the bond (heterolytic fission)
The halogen leaves the halogenoalkane as an X- ion
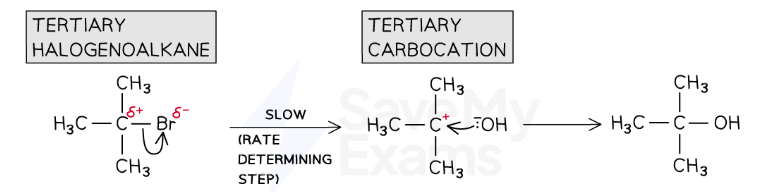
Sn1
Tertiary Carbocation
Two step process
n the first step, the C-X bond breaks heterolytically and the halogen leaves the halogenoalkane as an X- ion (this is the slow and rate-determining step)
This forms a tertiary carbocation (which is a tertiary carbon atom with a positive charge)
In the second step, the tertiary carbocation is attacked by the nucleophile
Reactivity of Halogenoalkanes