Orgo Chem Exam 1 Content
1/65
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
66 Terms
gen. name for 1 carbon compound?
methane
gen. name for 2 carbon compound?
ethane
gen. name for 3 carbon compound?
propane
gen. name for 4 carbon compound?
butane
gen. name for 5 carbon compound?
pentane
gen. name for 6 carbon compound?
hexane
gen. name for 7 carbon compound?
heptane
gen. name for 8 carbon compound?
octane
gen. name for 9 carbon compound?
nonane
gen. name for 10 carbon compound?
decane
gen. name for 11 carbon compound?
undecane
gen. name for 12 carbon compound?
dodecane
naming for alkenes?
drop “-ane” replace with “-ene”
naming for alkynes?
drop “-ane” replace with “-yne”
naming for alcohols?
drop “-ane” replace with “-nol”
naming for rings?
add “cyclo-” before name as prefix
naming for ethers?
add “ether” at the end
naming for aldehydes?
drop “-e” replace with “-al”
naming for ketones?
drop “-e” replace with “-one”
naming for carboxylic acids?
drop “-e” replace with “-oic acid”
alkane?
hydrocarbons with single C-C bond
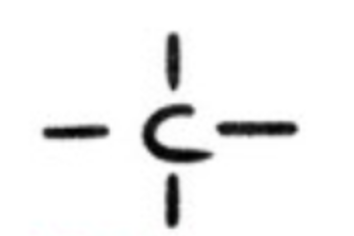
alkene?
hydrocarbons with double C-C bond
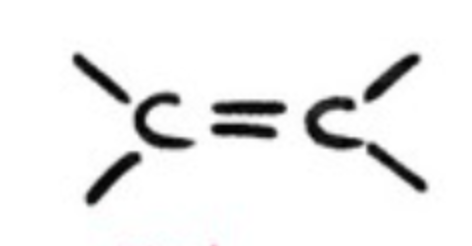
alkynes?
hydrocarbons with triple C-C bond
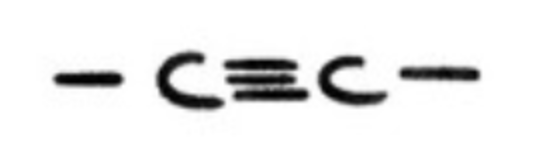
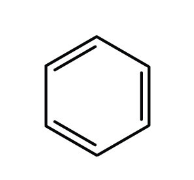
aromatic hydrocarbon?
derivative of benzene, 6 membered ring
alcohols?
organic compound containing (OH-) group
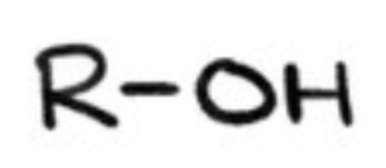
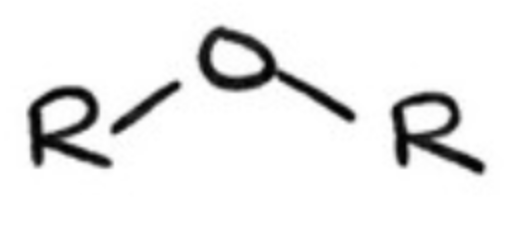
ethers?
composed of 2 alkyl groups bonded to O
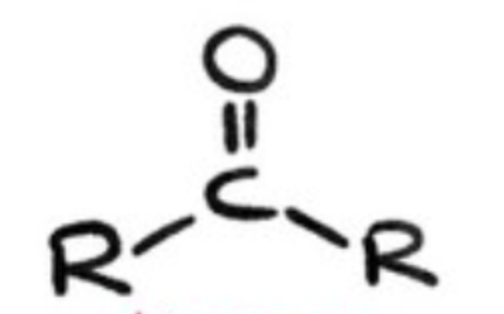
ketone?
2 alkyl groups bonded to 1 carbonyl group
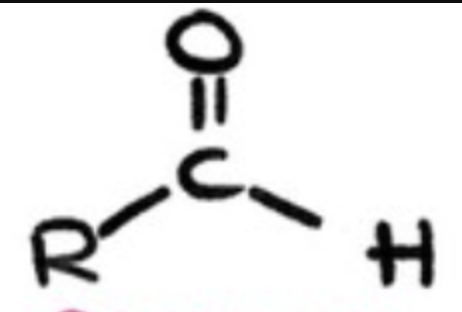
aldehyde?
1 alkyl group bonded to 1 carbonyl group
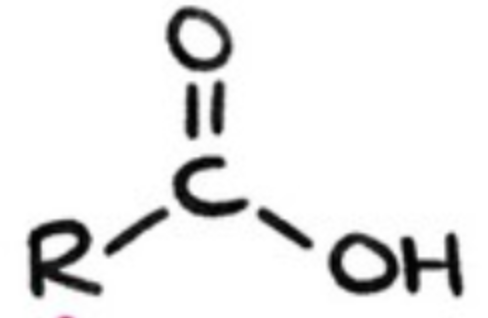
carboxylic acids?
contain carboxyl group -COOH
amines?
alkylated derivatives of ammonia
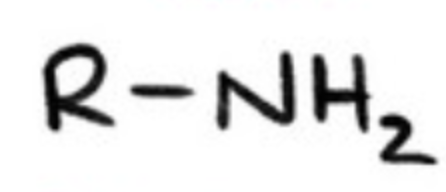
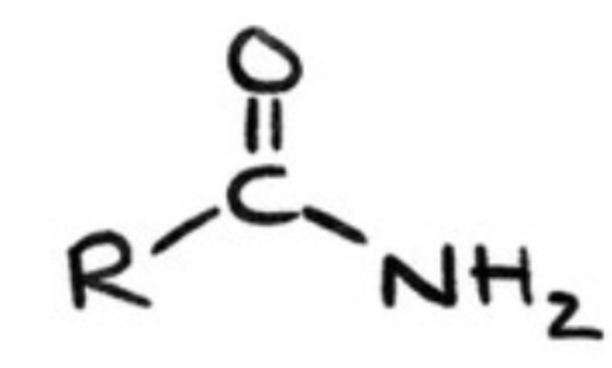
amides?
acid derivatives that result from a combination of an acid with ammonia or an amine
nitriles?
compound containing cyano group -C(triple bond)N
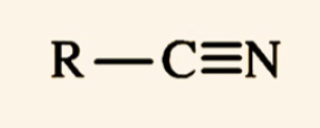
electrophile
Lewis Acids: species that can accept e- pairs
nucleophile
Lewis Bases: species that can donate e- pairs
as EN, ionic size, inductive effect, resonance stability ______… acidity ______
increases, increases
what is the stability order of hybridization?
sp3 < sp2 « sp
acetic acid pka?
4.76
water pka?
15.7
to convert pka to ka?
pka = -log(ka)
to convert ka to pka?
ka = 10^-pka
degree of alkyl substitution
just look at picture
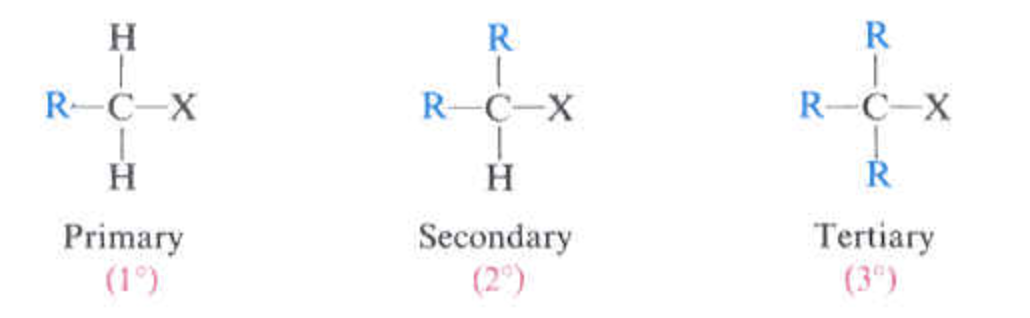
general formula for amount of C and H in an organic compound?
CnH2n+2
θ = 0
eclipsed conformation
θ = 60
staggered conformation
θ = anything else
skew conformation
θ = 0 for BUTANE
totally eclipsed
θ = 60 for BUTANE
gauche
θ = 120 for BUTANE
eclipsed
θ = 180 for BUTANE
anti
axial bonds
C-H bonds directed parallel to axis of ring
equatorial bonds
C-H bonds pointed out of ring
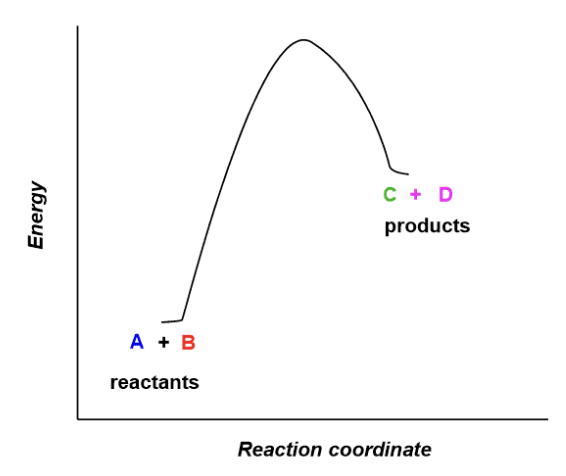
when products have greater energy than the reactants this reaction is considered ______
endothermic
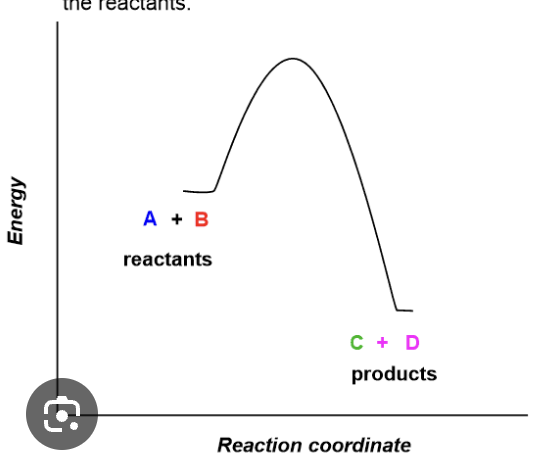
when reactants have greater energy than the products this reaction is considered ______
exothermic
initiation step
no radicals → 2 radicals
propagation step
1 radical + 1 product → 1 radical + 1 product
termination step
2 radicals → 1 product no radicals
homolytically breaking a bond
each bonded atom retains one of the bond’s 2 e-s
heterolytically breaking a bond
one atom retains both e-s
rate law
rate = kr[A]^a[B]^b
when concentration of reactant doubles and rate law doubles
first order
when concentration of reactant doubles and rate law quadruples
second order
when concentration of reactant doubles and rate law remains the same
zero order
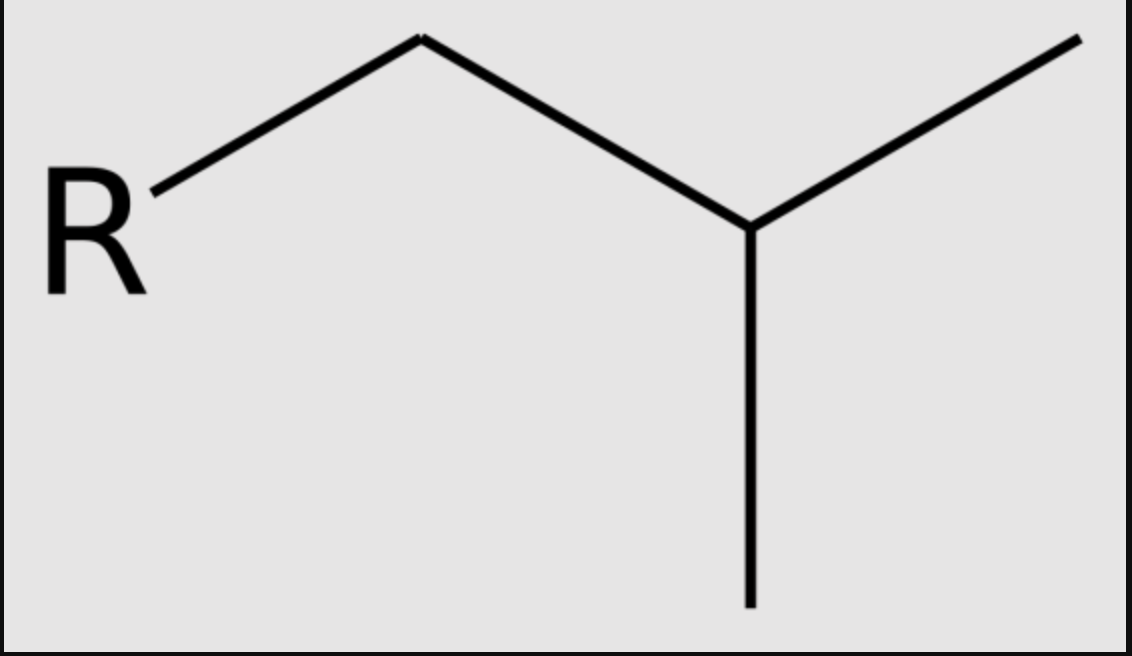
what is the special name of this group?
isobutyl
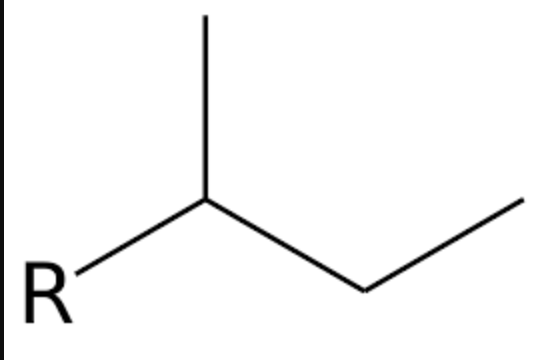
what is the special name of this group?
sec-butyl
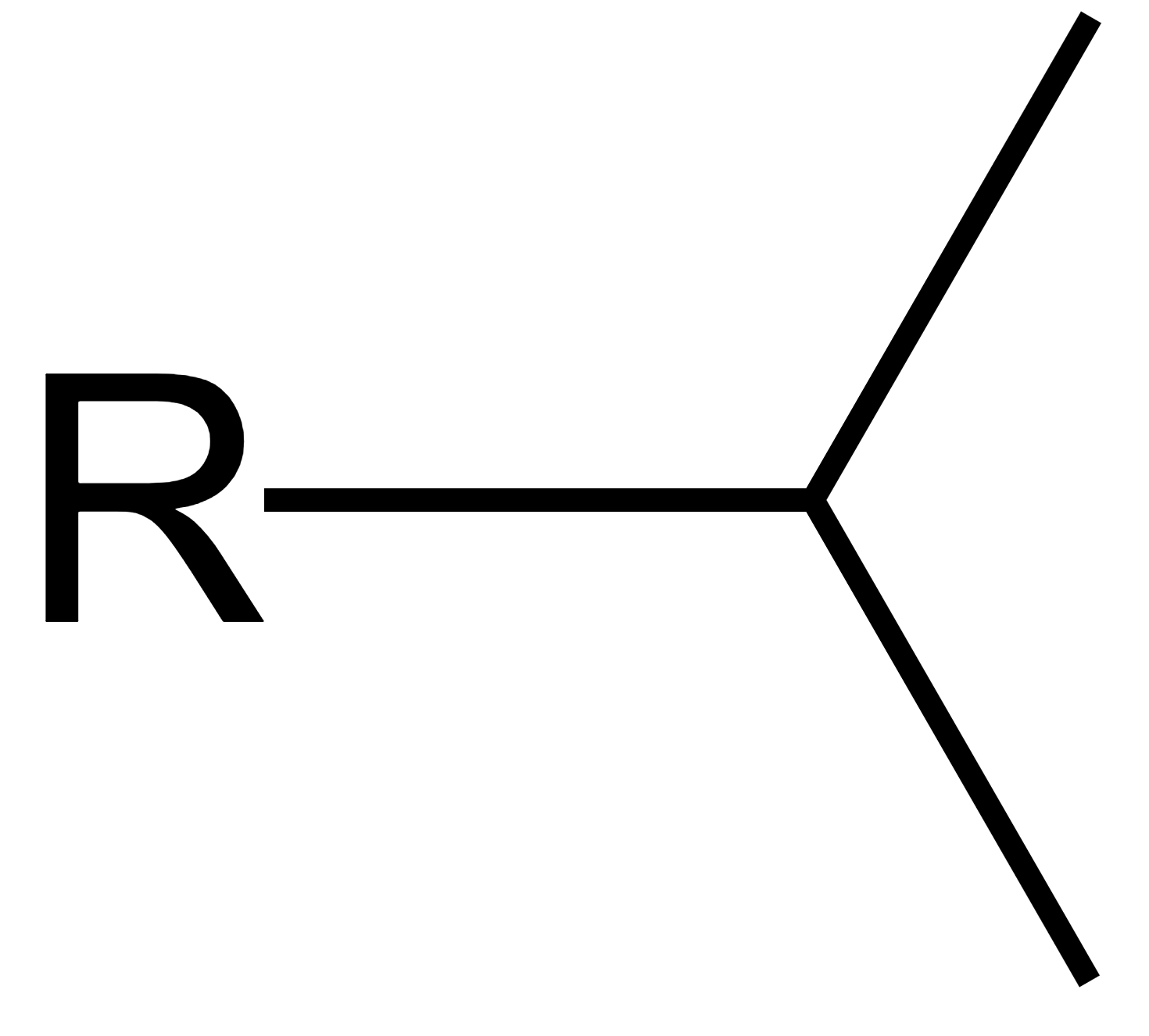
what is the special name of this group?
isopropyl
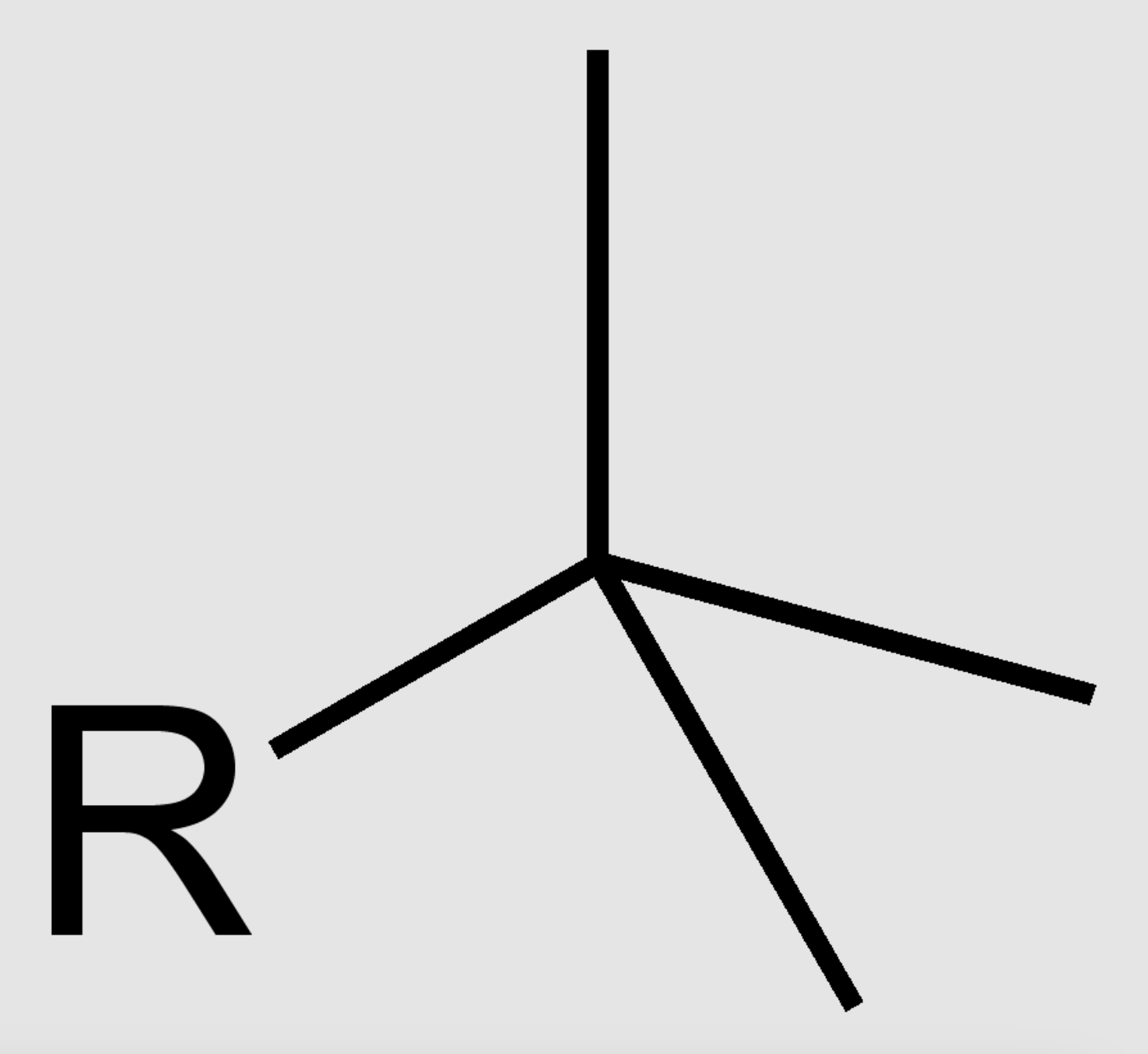
what is the special name of this group?
tert-butyl