Chapter 16 Aromatic Compounds and Reactions
1/25
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
26 Terms
Aromatic Coumpounds
Aromatic: pleasant aromas
Stable ring structure (ex: Benzene)
Benzene
carbon bonds have the same bond order (1.5)
can be resonance stabilized (3 PI BONDS NOT DOUBLE BONDS)
pi bonds because the bonds aren’t fixed (it would be called double if it were fixed)
sp3 hybridized
Benzene Stability (Benzene Hydrogenation)
Not stabilized like a regular alkene (won’t be oxidized)
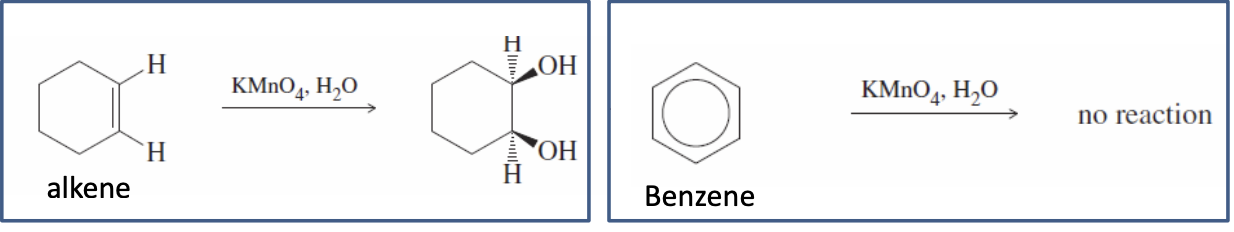
BUT IT CAN BE HYDROGENIZED (with a strong catalyst)
breaks its double bonds
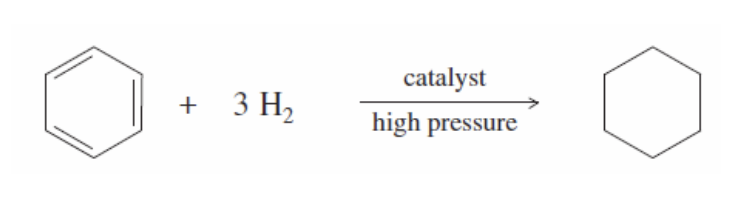
Annulenes
They are cyclic hydrocarbons that have non-fixed (alternating via resonance) single and double bonds
labeled by the number of carbons they have (ex: benzene = [6]annulene)
Not all are aromatic (pleasant smell)
aromaticity requirements: being cyclic & planar and follow 4n + 2pi rule
ex: cyclobutadiene and cyclootatetraene aren’t aromatic
Aromaticity Requirements
Structure must be cyclic w/ conjugated pi bonds (bonds separated only be 1 C)
each atom must have unhybridized p orbital
all p orbitals must overlap continuously
planar ring that is more stable than its open-chain counterpar

Cyclic (forms a ring)
Planar (flat, so p orbitals can overlap)
Fully conjugated (every atom in the ring has a p orbital)
Follows Hückel’s Rule: has 4n + 2 π electrons
Antiaromatic Compounds
Structures that are cyclic, conjugated, overlapping p orbitals BUT ITS OPEN-CHAIN COUNTERPART HAS GREATER ENERGY (MORE STABLE)
follow rules or aromaticity but are unstable
Nonaromatic Compounds
Structures that are not a continuous ring, does not have overlapping p orbitals, and can be nonplanar
do not follow aromaticity rules
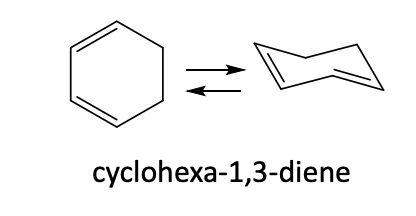
Hückel’s Rule
Aromatic: A ring is aromatic if it has a
continuous ring of overlapping p orbitals (fully conjugated)
planar structure
4n + 2pi electrons
ex: 2, 6, 10
They are very stable
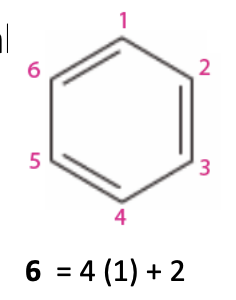
Antiaromatic: A ring is antiaromatic if it has a
continuous ring of overlapping p orbitals (fully conjugated)
planar
4n pi elecetrons
ex: 4, 8
They are very unstable (avoid total instability by usually becoming nonplanar)
Larger [N] annulenes aromoticity or antiaromoticity depends on if its ring can stay planar
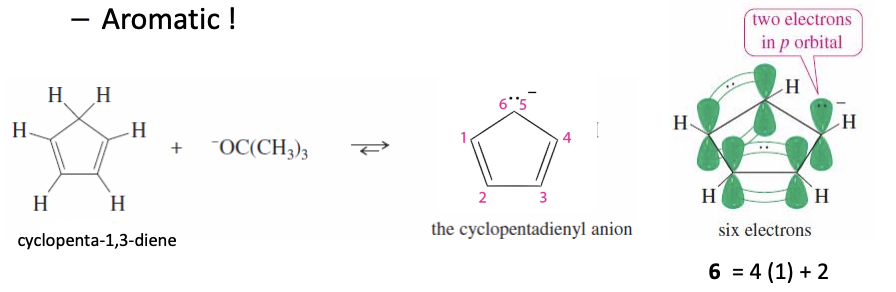
Cyclopentadienyl Anion
Has a nonbonding pair of e- in a p orbital
has 6 conjugated e-
it is AROMATIC (forming the anion is favorable so it is much more acidic than typical alkenes)
6 pi —> aromoatic —> stable
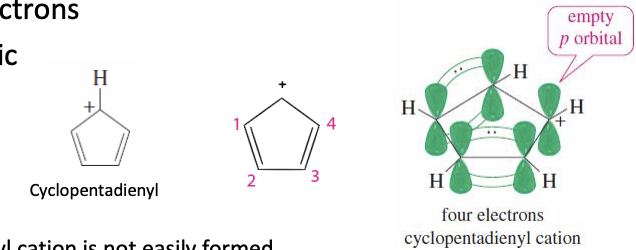
Cyclopentadienyl Cation
Has an empty p orbital
total 4 pi electrons (fits antiaromatic Hucks rule 4n)
ANTIAROMATIC (very unstable so it is not easily formed)
4 pi —> antiaromatic —> very unstable
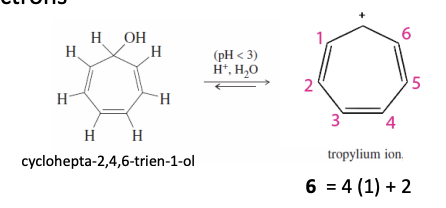
Cycloheptatrieneyl Ion
Tropylium Cation and Tropylium Anion
Tropylium Ion Cation
Has an empty p orbital
has 6 pi e- (4n + 2pi)
aromatic
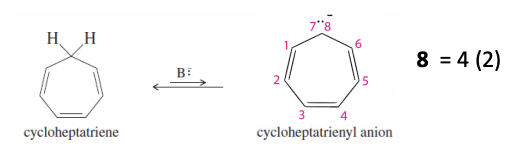
Tropylium Ion Anion
Has a nonbonding pair of e- in a p orbital
has 8 pi e
anti-aromatic
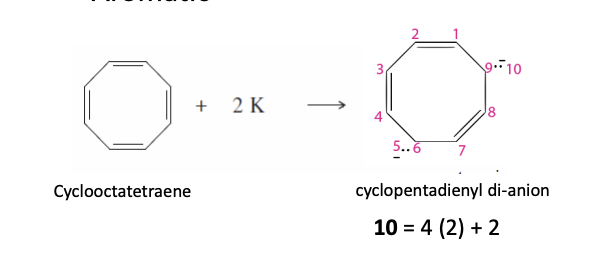
Cyclooctatetraene Dianonion
Has 2 non bonding pairs of electrons
has 10 pi electrons
Aromatic
MAIN Heterocyclic Aromatic Coumpounds
Pyridine and Pyrrole
also has Pyrimidine, Imidazole, Furan, and Thiophene
Pyridine
The nitrogen analog of benzene (benzene but nitrogen replaces one carbon)
6 pi e- —> aromatic —> stable
nitrogen has lone pairs (don’t overlap with the aromatic pi system)
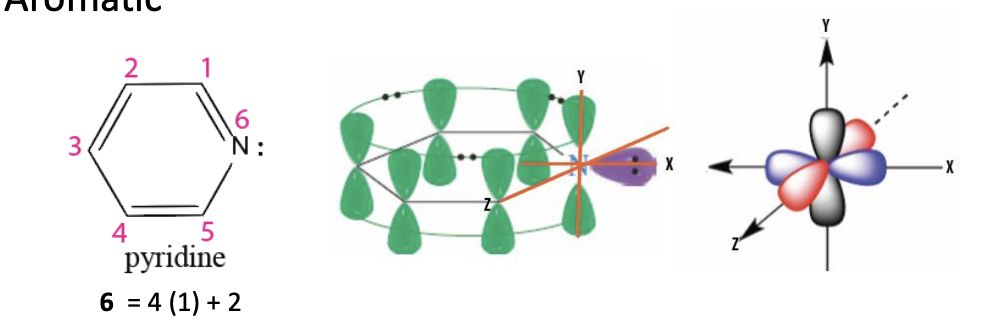
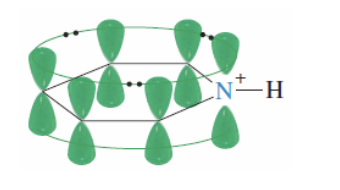
if protonated —> becomes pyridinium ion that is still aromatic
PROTONATED STILL AROMATIC
Pyrrole
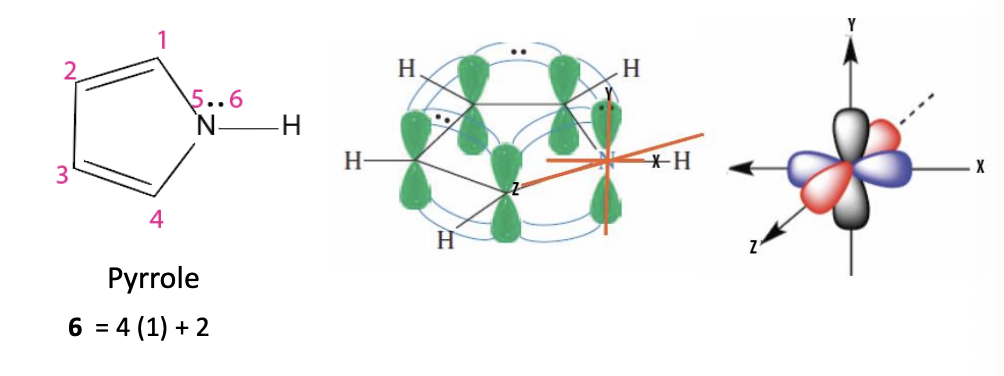
5 membered heterocycle ring (with nitrogen replacing a carbon, the N is conencted to an H)
6 pi e- —> aromatic —> stable
nitrogen lone pairs does overlap with the aromatic pi system
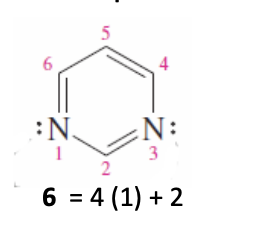
Pyrimidine
6 membered ring with 2 nitrogens (benzene with 2 N’s instead of c’s)
N’s lone pairs don’t overlap the aromatic pi system
6 conjugated pi e- = aromatic = stable
Imidazole
5-membered ring with 2 nitrogens (1 nitrogen is NH)
one of the lone pairs of the Ns (the NH) DO OVERLAP with the aromatic pi system
the other N doesn’t
6 pi e’s —> aromatic —> stable
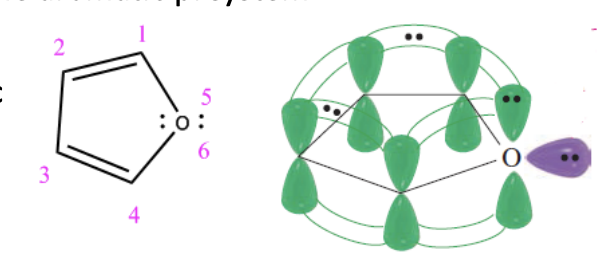
Furan
5-membered ring with OXYGEN
2 lone pairs do overlap with the aromatic pi system
ONE OF THE PAIRS OVERLAP, the other doesn’t
6 conjugated pi e’s —> aromatic —> stable
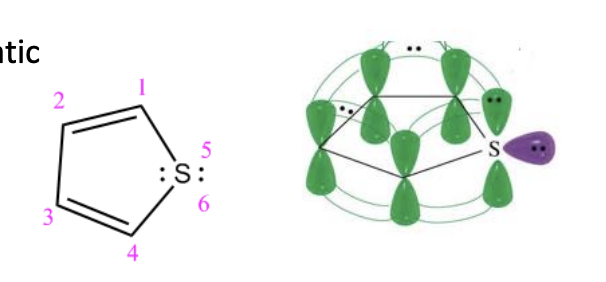
Thiophene
5 membered ring with SULFUR
2 pair of lone pairs
ONE OF THE PAIR OVERLAPS THE PI SYSTEM, the other doesn’t
6 conjugated pi e’s —> aromatic —> stable
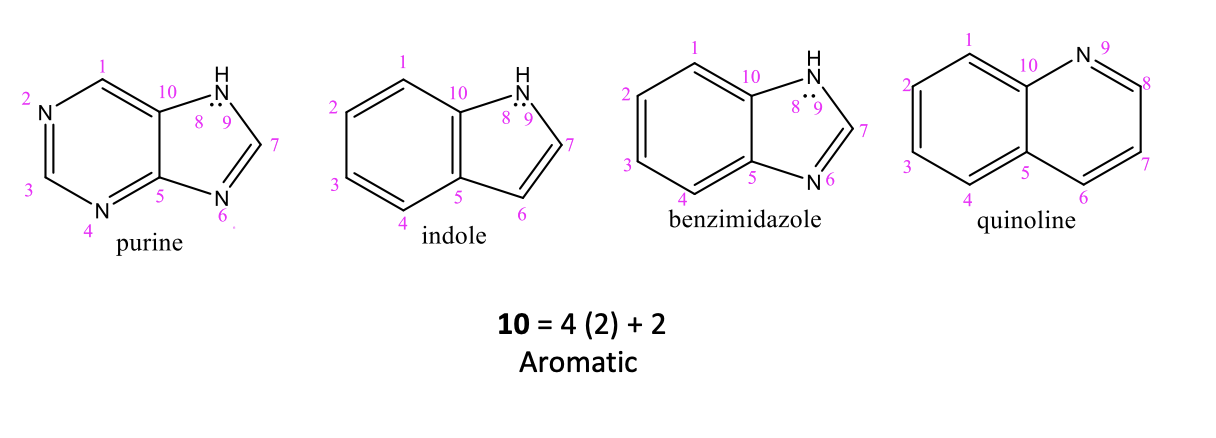
Fused Heterocylic Compounds
When heterocyclic compounds are fused together, forming fused-ring heterocycles
they are similar to the simple heterocycles
THEY MUST FORM 10 π electrons (THEY ARE STILL AROMATIC)
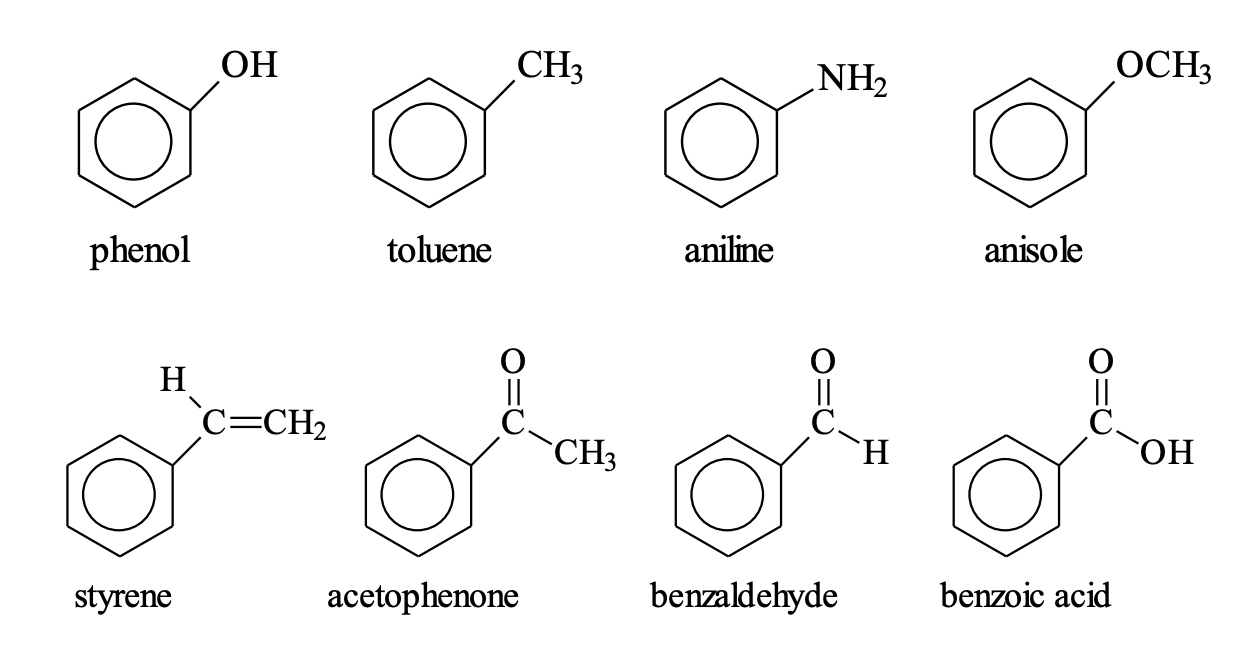
Benzene Derivatives Common Names
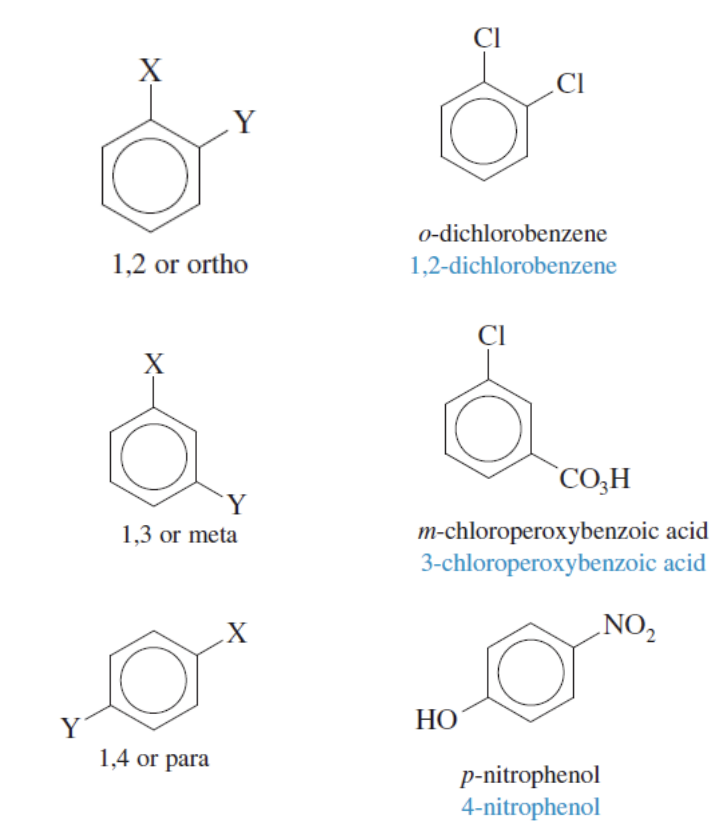
Disubstituted Benzenes (Positions)
There is Ortho, Meta, and Para
Ortho: 1,2 pos
Meta: 1,3 pos
Para: 1,4 pos
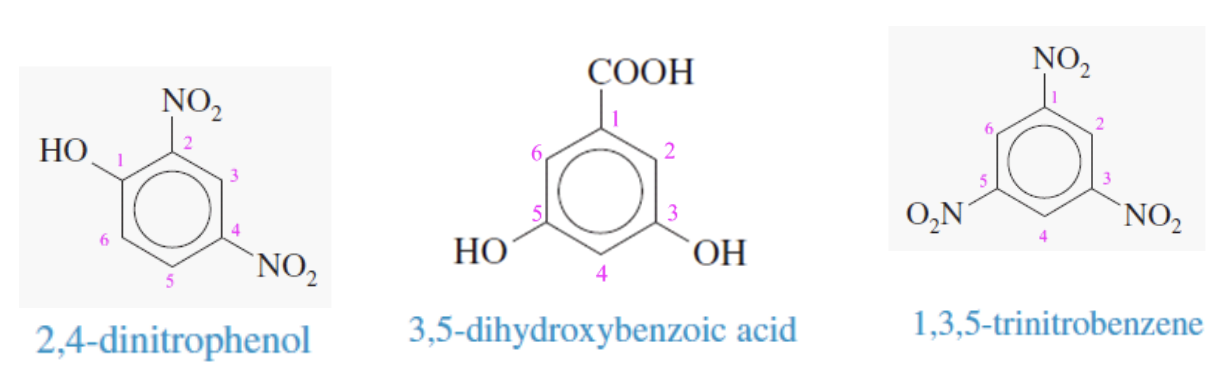
Benzene with 3 or more substituents naming
Carbon with the root substituent (phenol or benzoic acid, OH or COOH) gets labeled pos 1
the rest are numbered to give the lowest possible number to substituents
if both phenol OH and benzoic acid COOH are the substituents, benzoic acid COOh takes 1st priority
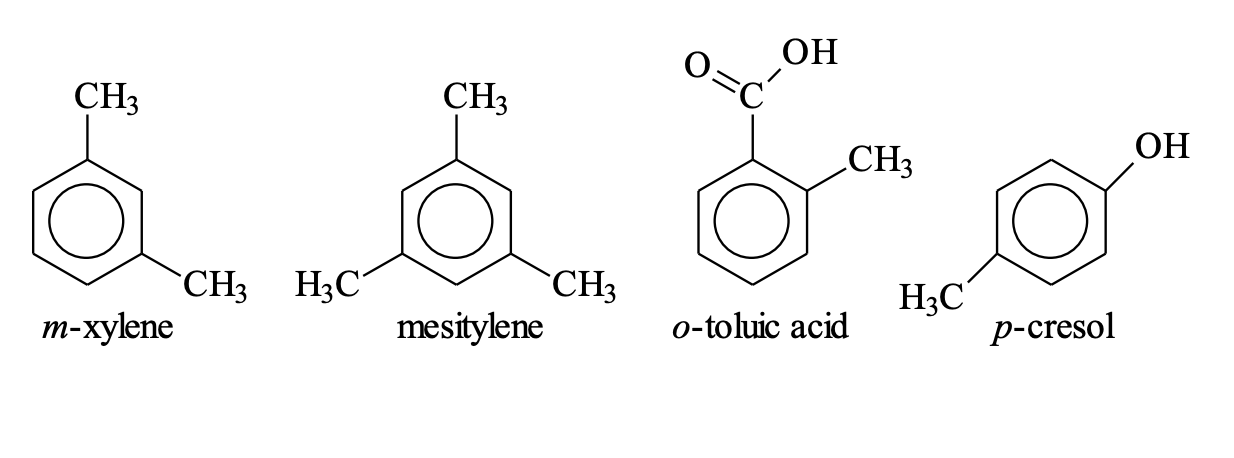
Common Names for Disubstituted Benzenes
Phenyl and Benzyl Group Substitued
Phenyl Group: when the benzene ring is the substituent on another group
benzene (on the ring) —> attaches to another molecule
Benzyl Group: when the benzene ring is attached to a methyl group (benzene + methyl that is attached to rest of another group)
benzene + CH2 —> attaches to another molecule
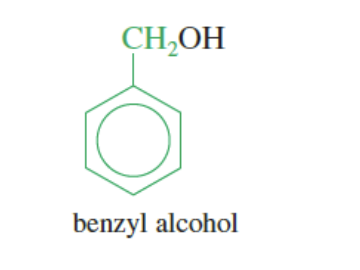
DIFFERENCE IS THE PHENYL GROUP ONLY HAS BENZENE AS THE SUBSTITUTENT WHILE BENZYL GROUP HAS A BENZENE +CH2 AS THE SUBSTITUTENT