chem unit 1 pt 9
1/11
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
12 Terms
chemistry
happens as atomes join in chemical bonds
represented by chemical formulas
the subscript to the right of the symbol of an element tells the number of atoms of that element in one molecule of the compound
empirical formulas
give the lowest whole-number ratio of atoms of each element in a compound
ex. CH
molecular formulas
give the actual number of atoms of each element in a compound
if we have the ____ of a compound, we can determine it’s empirical formula. The reverse is not true without more information
ex. CH C2H2 C6H6
chemical bonds
always involve the sharing of electrons between 2 or more nuclei
three types
covalent or molecular compounds
ionic compounds
metallic systems
they are loosely defined by the way electrons are “shared” between the constituent atoms
ion
an atom that gains or loses electrons
cations (+)
anions (-)
cation
formed when 1 or more electrons are lost
positive (more protons than electrons)
anion
formed when 1 or more electron is gained
negative (more electrons than protons)
ionic charge
shown by a superscript to the right of the atomic symbol
ex. Ca²+ (Ca^2+)
polyatomic ions
formed from compounded atoms gaining or losing 1 or more electrons
ex. polyatomic cation - 4(NH^+)
covalent compounds
electrons are close to evenly shared between 2 atoms that are bonded
X represents an electron “from” hydrogen
circle represents an electron “from” carbon
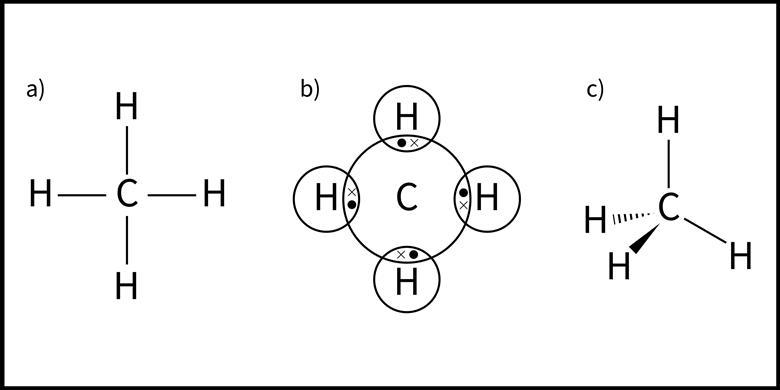
ionic compounds
are generally formed between metals and nonmetals
electrons are transferred from the metal to the nonmetal
the oppositely charged ions attract each other
only empirical formulas are written
ex. NaCl
metals
good conductors because some electrons are shared across many nuclei
kind of like large scale plum pudding