atomic structure
1/44
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
45 Terms
Thomson
1897 found the electron
nicknamed the “plum pudding”
Rutherford
1918 found the proton
also gold foil experiment
chadwick
1932 found neutron
electron location
empty space (electron cloud)
proton location
in nucleus
neutron location
in nucleus
electron actual mass
9.1 × 10 e -28 g
proton actual mass
1.672 × 10 e -24 g
neutron actual mass
1.674 × 10 e -24
electron amu
0.0006
proton amu
1.00
neutron amu
1.00
electron charge
-1
proton charge
+1
neutron charge
0
Bohr
1913- attempted to explain the location of the electron
Isotopes
atoms with the same identity but different mass.
same number of protons
different number of neutrons
atomic emission Spectra
set of frequency of waves emitted by atoms
electrons
naturally in a ground state (lowest energy)
closest to the nucleus
Democritus
matter is composed of tiny particles called atoms
Aristotle
disagreed with the idea of atoms
believed in the 4 elements
Define atomic number
the number of protons in the nucleus.
H. Mosley discovered each element contains a unique positive charge.
determines the place for each element on the periodic table.
mass number
total number of protons and neutrons found in the nucleus.
REMEMBER! ALL of the mass of the atom is found in the nucleus
BAND-AID POINT
internal electrons remain the same
cd[kr]5s²4d^10
electron dot
only highest level electrons as a dot value electrons.
8 maximum-called an octet can be predicted by location
electron configuration
number of electrons in the sublevel as a transcript
1s² 2s² 2p^4
orbital notation
shows every electron with an arrow
P orbital
“dumbbell orbital” duel loaf always opposite
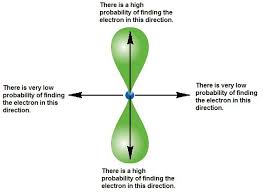
S orbital
spherical orbital
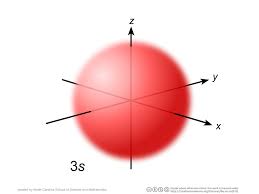
F
7 spots can hold 14
D
5 spots can hold 10
P
3 spots can hold 6
S
1 spot can hold 2
wavelength
Lamba usually expresses in m,cm,nm
frequency
number of waves that pass a point per second or given in time
speed
C- 3.00×10^8 m/s
C is a constant so it cant be calculated
all electromagnetic waves travel at the speed of light
X-ray
penetrate the skin only, it cant go any deeper to the bones
cosmic rays
because they're shorter they have a higher energy level
gamma rays
often used in cancer treatments more frequently brain cancers
transverse wave
travel at the speed of light
electron configurations
1.) The arrangement of electrons in an atom.
2.) lowest energy and most stable.
3.) follows 3 basic rules of predicting arrangement.
rules of electron arrangement 1.)
aufbau principle- each e occupies the lowest energy orbital.
Each sub level has a different energy state
e^- within an energy level fill in sub level order S,P,D,F
The energy levels overlap so a guideline is needed to establish sub level order
rules of electron configuration 2.)
pauli exclusion principle- an atomic orbital contains a maximum of 2 e^-
the 2 e^- will travel with opposite spins
direction of spin will be represented by the arrows
rules of electron arrangement 3.)
Hunds rule
e^- will individually occupy equal energy orbitals before forming a pair.