Acid-Base Review
1/36
Earn XP
Description and Tags
POP 2 Exam 2
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
37 Terms
What are the two types of acids produced by the body on a daily basis?
Metabolic (organic / non-volatile) acids and CO2
Which acid is greater?
PaCO2 is much greater – we have an acid load to remove daily – body handles low pH better than high pH
How are they removed from the body?
Metabolic (organic / non-volatile) acids (removed via kidneys)
CO2 (removed via lung – fast and kidneys – slow)
What are the processes by which pH is maintained constant in the body?
Tissue and plasma buffers
Lungs
Kidneys
How quickly do these processes work?
Tissue and plasma buffers (fastest , but limited)
Lungs (control CO2 – fast)
Kidney (slow, but powerful)
How does the normal daily acid production compare to the actual amount of H+ present in the serum?
Productions is much greater than the plasma pool; we have to get rid of it on an ongoing basis
What are the key buffer systems in the body and where do they play their key role?
Bicarbonate: extracellular fluid
Phosphate: renal tubule and intracellular
Ammonia: renal tubule
Proteins: intracellular and plasma – These buffer systems have overlapping ranges to allow a cumulative buffer capacity – allows control over a greater pH range
Name one key protein buffer.
Hemoglobin
What is unique about the protein buffers?
Can act as an acid or base (amphoteric)
What are the key characteristics of the bicarbonate buffer system?
Reversible equation (carbonic anhydrase) drive by the relative concentration of the substrates
Open system since we can quickly excrete CO2 via the lungs
Can also regulate HCO3 and H+ via the kidneys
Normal ratio of HCO3:CO2 is 20:1 which yields a pH of 7.42 (Henderson-Hasselbach equation)
What does hyperventilation do to pH?
Hyperventilation --> decreased PaCO2 --> increased pH
What does hypoventilation do to pH?
Hypoventilation --> increased PaCO2 --> decreased pH
How much can changes in ventilation impact pH?
limited change of approximately 0.2 – limited effect on ventilation until pH changes by greater than 0.2 or more
What roles do the kidneys play in regulation of acid-base balance?
Slower but powerful; Eliminate sulfate and phosphate acids
What cells are responsible for “fine-tuning” acid-base balance and where are they located?
alpha-intercalated cells – secrete H+ can change the number of H+ ATPase pumps over time as needed;
beta-intercalated cells – reabsorbs H+, secretes HCO3-
Both located in distal tubule
Where is most hydrogen ion secreted in the tubule?
Proximal tubule is the primary site
Where do phosphate and ammonia act as a buffer in the tubule?
Ammonia buffers in the proximal, ascending LOH, and distal tubule; phosphate is important proximally, bicarbonate is important proximally
What happens to pH as filtrate moves along the tubule?
pH is acidic proximally, rises as it travels down LOH (concentration of bicarb) then becomes acidic again by distal tubule. Urine is acidic.
How is ammonia produced by the kidney?
Glutamine can be metabolized to ammonia
What is one mechanism by which the kidneys can consume H+?
alpha-ketoglutarate can be converted to glucose using a hydrogen ion.
Where is most bicarbonate reabsorbed?
Proximal tubule
How is bicarbonate reabsorbed?
Converted to CO2 which diffuses into tubule cells where it is converted back to bicarb. Hydrogen ion is secreted in this process.
How does the body get rid of phosphate acids?
The hydrogen ion from bicarbonate reabsorption enters the filtrate in the tubule via an Na/H antiporter. Reacts with the phosphate to “trap” it in the filtrate for excretion. Process results in loss of phosphate acid
What are 6 ways the body can change hydrogen ion secretion?
Low intracellular pH increases the number of H+ transport pumps
Increased PaCO2 converted to H+
Increased carbonic anhydrase activity
Hypokalemia (distal tubule loss of H+ as potassium is reabsorbed)
Aldosterone
Increased sodium reabsorption distally
Can you interpret the arterial blood gas for the various acid-base disorders?
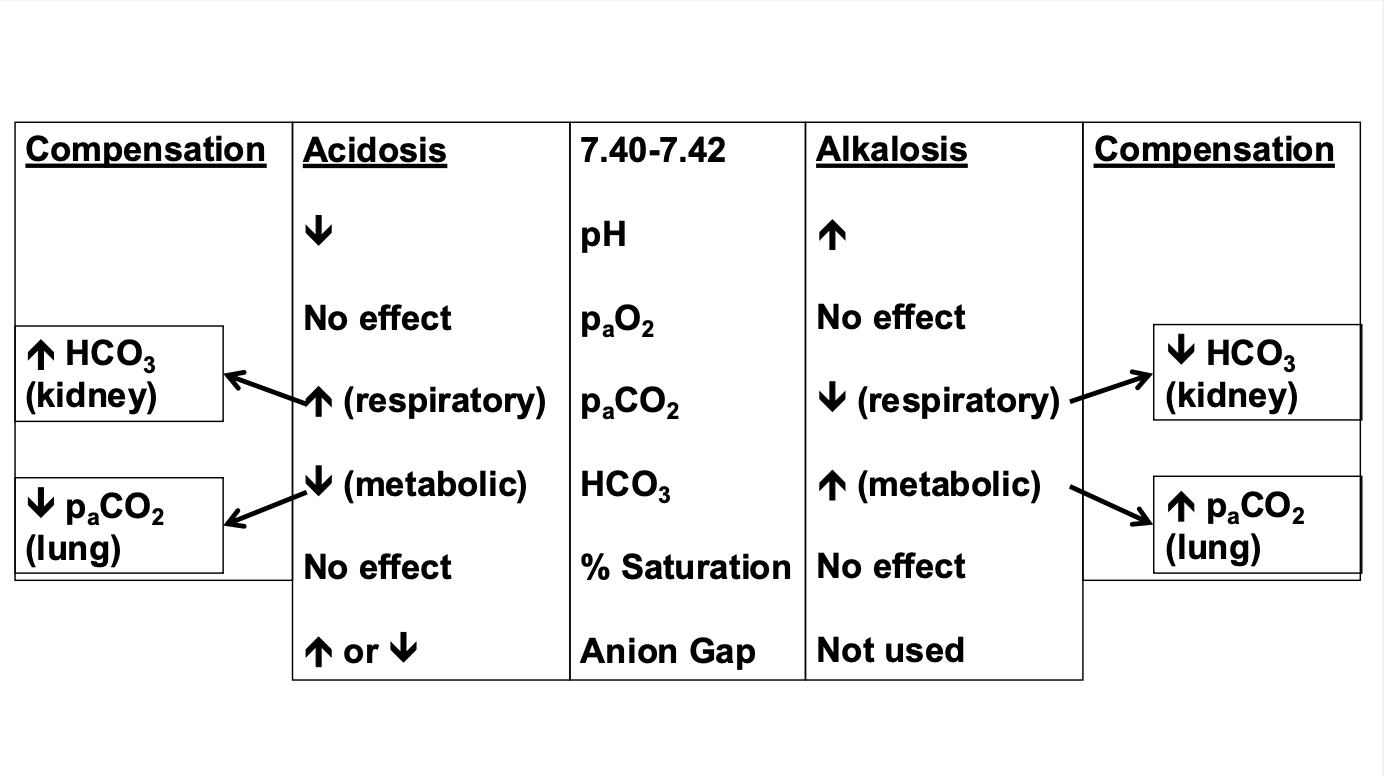
How does the body compensate for Respiratory Acidosis?
Respiratory acidosis (inc. PaCO2) – kidneys will secrete H+ and retain HCO3
How does the body compensate for Respiratory alkalosis?
Respiratory alkalosis (dec. PaCO2) – kidneys will retain H+ and secrete HCO3
How does the body compensate for metabolic acidosis?
Metabolic acidosis (dec. HCO3) – lungs increased ventilation to decrease PaCO2
How does the body compensate for metabolic alkalosis?
Metabolic alkalosis (inc. HCO3) – lungs decreased ventilation to increase PaCO2
What are common causes of respiratory acidosis?
Hypoventilation or lung diseases that impact gas exchange
damage to the medulla
drugs that depress the breathing centers (opiates)
What are common causes of metabolic acidosis?
Chronic renal failure, diarrhea, biliary vomiting, ketoacidosis, methanol
What are common causes of respiratory alkalosis?
Panic disorders, hypoxia (altitude)
What are some causes of metabolic alkalosis?
Hyperaldosteronism, gastric vomiting
How do you calculate anion gap?
Anion gap = Sodium – serum bicarbonate – Chloride
normal gap is the unmeasured anions (mainly plasma proteins)
When is anion gap used?
used to differentiate the type of metabolic acidosis
What are some normal gap acidosis?
Diarrhea, renal tubule dysfunction
What are some causes of metabolic acidosis associated with an increased anion gap?
carbonic anhydrase inhibitors; ammonium chloride ingestion