BME 452 Exam 2
1/38
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
39 Terms
Deformation
change in shape sufficient to cause loss of function
Types of Deformation
Time Independent
elastic
plastic
buckling
Time Dependent
creep
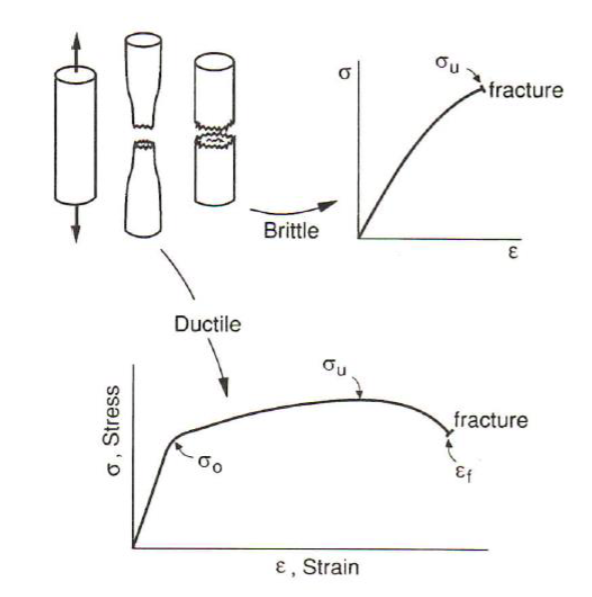
Fracture
cracking into two or more pieces
Types of Fracture
Static Loading
brittle
ductile
rupture
Dynamic Loading
fatigue
crack growth
Most Common Biomaterial Failure Modes
mechanical failure (fracture, fatigue, wear)
corrosion and chemical degradation
biological reaction
design and manufacturing choices
poor biocompatibility/immune response
infection related failure (biofilm)
Elastic Deformation Diagram
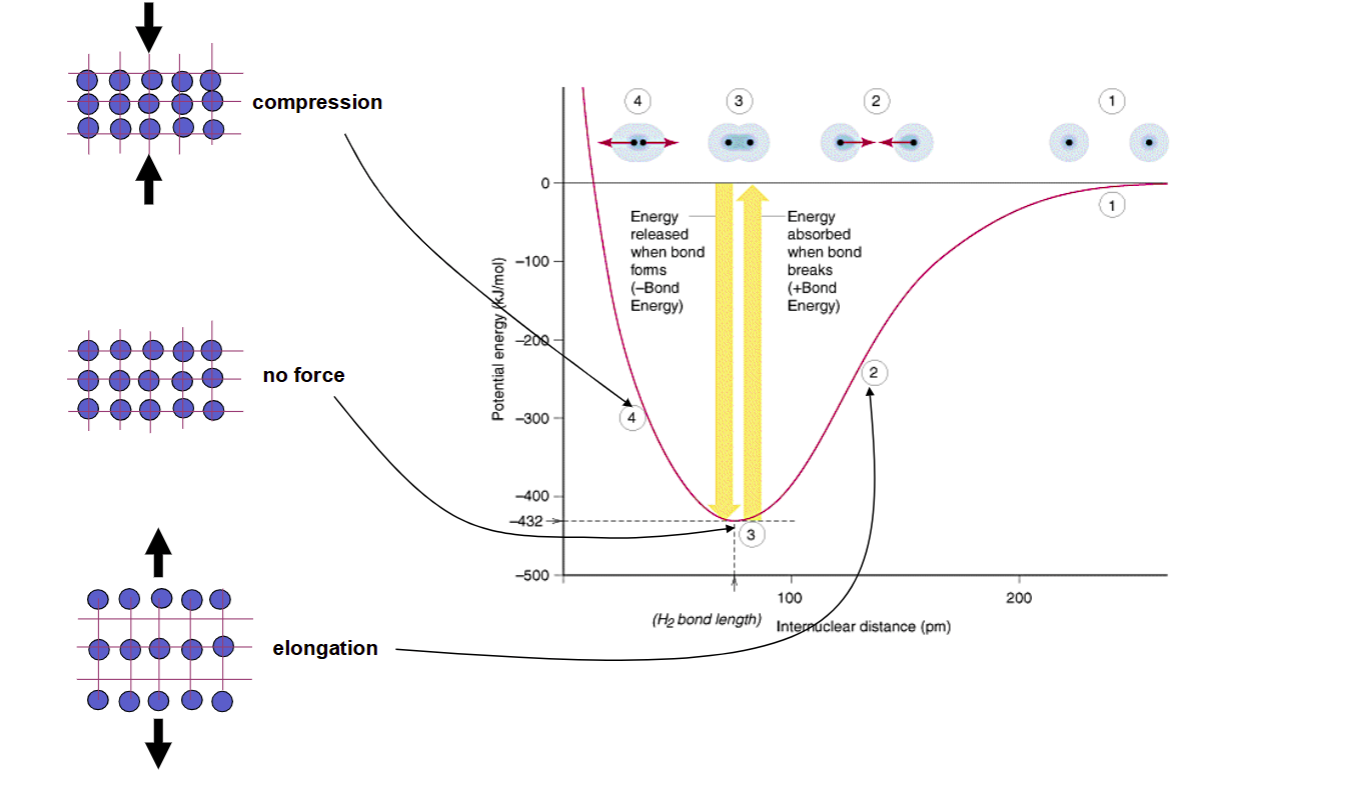
Elastic vs Plastic
Elastic
stretching of chemical bonds
Plastic
rearrangement of atoms or lattice structure
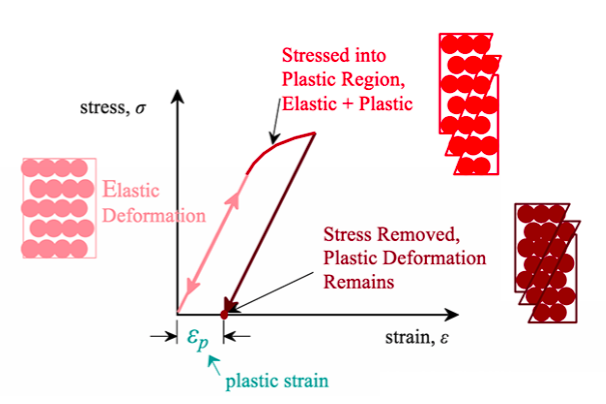
Plastic Deformation Occurs by
dislocation motion
diffusion of vacancies
sliding along grain boundaries
Plastic Deformation Graph
Fracture Graph
Fatigue
progressive and localized structural damage that occurs when a material is subjected to repeated cyclic loading or stress
Safety Factor
X = failure life/desired service life
Safety Factor for Fatigue
X fatigue = # cycles to failure/# cycles in service
Creep Deformation
gradual and time-dependent deformation of a material that occurs under constant load or stress at elevated temperatures
Example of how defects in crystal can alter its properties
increases conductivity of semiconductors
changes colors of insulators and glass
uptake of small atoms such as hydrogen
Types of defects in crystal
point defects, line defects, and planar defects
Point Defects
vacancy
self interstitial
interstitial impurity atom
substitutional impurity atom
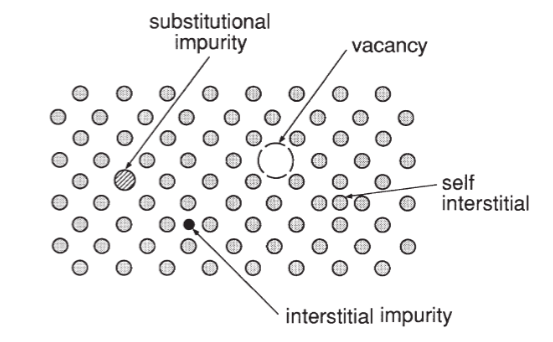
Volume Defects
large macroscopic vacancies or precipitates in material
Volume defects good and bad
Good:
reduce weight
serve as filter
voids where cells may interact with material
greater bonding strength of surface
Bad:
reduced mechanical properties
stress concentrations
permeable to water
Porosity = Vp = 1 - Vs (volume fraction of solid phase)
Mechanisms of plastic deformation in metals
slip and twinning
which plane is most likely to promote slippage?
one with highest planar densities
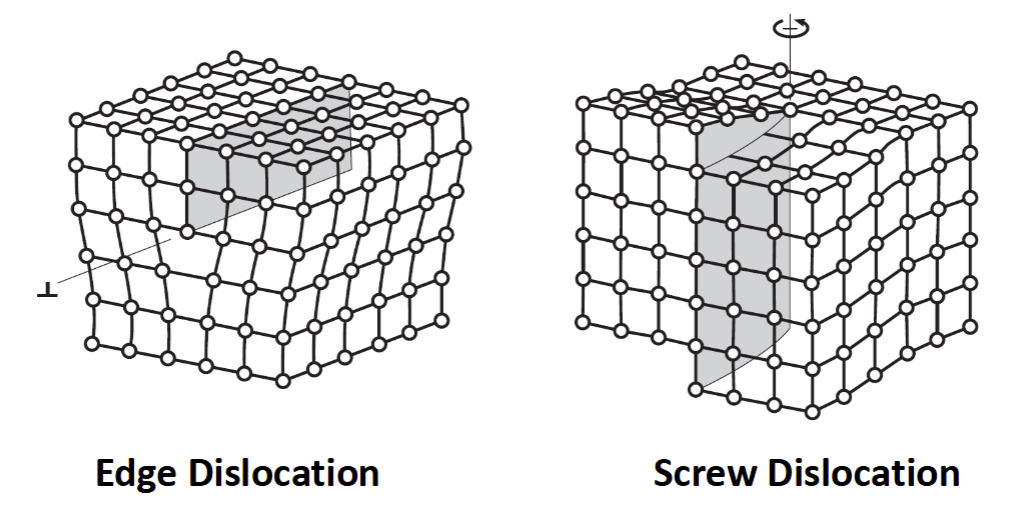
Dislocations (Linear Defects)
linear defects in the material crystal structure
extra half-plane of atoms present
localized lattice strains
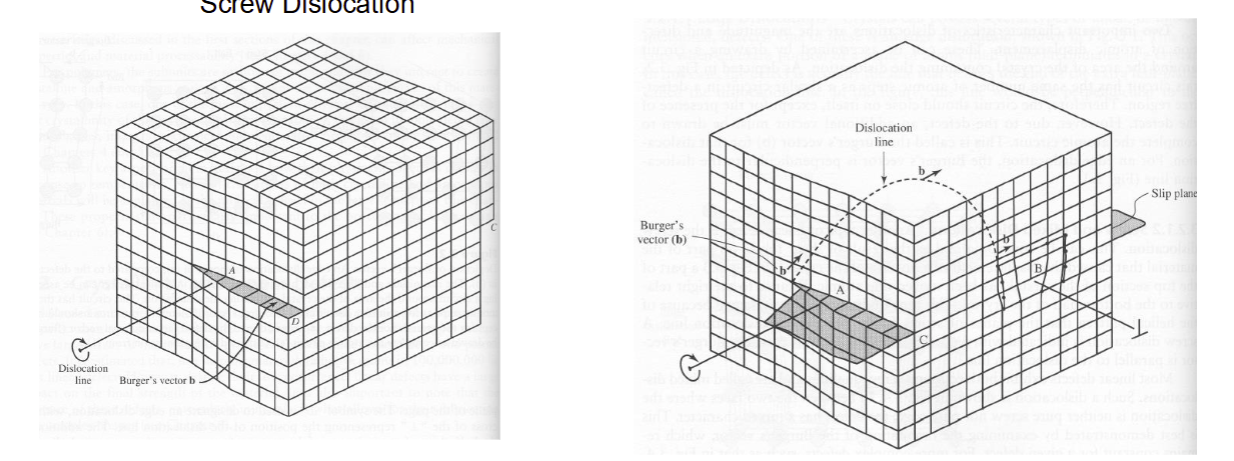
Dislocation Motion
move throughout crystal structure
one atom wide, glides through the crystal
glide along the atomic planes int he direction of the Burger’s vector
shear stresses move dislocations
Two Basic Types of Dislocations
edge dislocation and screw dislocation
Screw and Mixed Dislocations
where does slip and climb occur?
at dislocations
where does slide of atoms occur?
grain boundaries
What happens when a dislocation reaches another dislocation?
Annihilation: Dislocations with opposite signs ($\vec{b}$ and $-\vec{b}$) on the same plane attract and cancel out, restoring the lattice.
Repulsion & Pile-up: Dislocations with the same sign on the same plane repel each other. This causes "traffic jams" (pile-ups) that increase the stress needed for further deformation.
Intersection (Jogs/Kinks): When they cross different planes, they "nick" each other:
Jogs: Steps that move the line out of the slip plane (high resistance).
Kinks: Steps within the slip plane (low resistance).
Reaction: They may combine to form a new dislocation if it reduces total energy (if $b_{new}^2 < b_1^2 + b_2^2$).
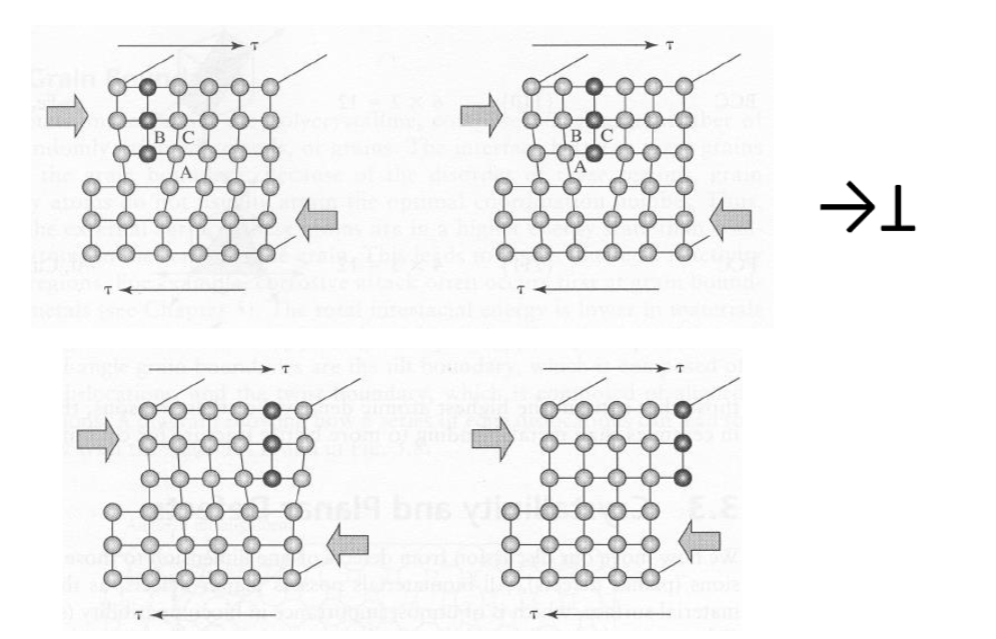
What happens when a dislocation reaches the end of the crystal (grain)?
1. At a Free Surface: Surface Steps
When a dislocation reaches the external edge of a crystal, it exits the lattice entirely.
The Result: It creates a microscopic "ledge" or surface step exactly one Burgers vector ($\vec{b}$) in height.
The Physics: The dislocation is annihilated because it no longer exists within the bulk. While this reduces the internal strain energy of the crystal, it slightly increases the surface energy by creating new surface area.
2. At a Grain Boundary: Obstruction & Pile-up
Grain boundaries act as powerful walls because the atomic planes in the neighboring grain are tilted at a different angle.
The Obstruction: The dislocation cannot easily "jump" into the next grain because its slip plane and Burgers vector don't align with the new crystal's orientation.
The Pile-up: Subsequent dislocations moving on the same plane get stuck behind the first one, like a traffic jam at a toll booth. This is called a dislocation pile-up.
Stress Concentration: The pile-up creates a massive local stress field. Eventually, this stress may become high enough to "activate" a new dislocation source in the neighboring grain.
3. The Big Picture: Grain Refinement
This interaction is the basis of the Hall-Petch Effect.
Smaller Grains = More Boundaries: More boundaries mean more obstacles for dislocations.
Strength: Because the dislocations are pinned and cannot move easily, the material becomes much stronger and harder.
How would grain size affect dislocation motion?
Grain size is essentially the "length of the runway" for a dislocation. The smaller the grain, the shorter the runway, and the harder it is for the material to deform.
Here is how grain size dictates the behavior of dislocations:
1. The Grain Boundary as a Barrier
A grain boundary is the interface where two crystals with different orientations meet. Dislocations move along specific planes and directions (slip systems). When a dislocation hits a grain boundary, it stops because:
Disorientation: The slip plane in Grain A doesn't line up with the slip plane in Grain B.
Disorder: The boundary is a region of atomic mismatch, making it energetically "expensive" for the dislocation to pass through.
2. The Mean Free Path
The "mean free path" is the distance a dislocation can travel before hitting an obstacle.
Large Grains: Dislocations can travel long distances, build up speed (metaphorically), and require less external stress to move through the bulk of the grain.
Small Grains: The density of barriers is much higher. A dislocation can only travel a tiny distance before it’s pinned against a wall. This significantly restricts overall plastic flow.
3. The Power of the Pile-Up (The Hall-Petch Effect)
This is the most critical part. When dislocations get stuck at a grain boundary, they form a pile-up. This pile-up acts like a magnifying glass for stress:
In a large grain, you can fit many dislocations into one pile-up. The combined stress field of all those dislocations "pushes" on the grain boundary, making it easier to trigger a new dislocation in the next grain.
In a small grain, there isn't enough room for a big pile-up. With fewer dislocations pushing, you need much more external force to get the deformation to move into the neighboring grain.
Grain Boundary
the interface of neighboring grains
atoms on edge of grain have a …
higher energy state and higher chemical reactivity (ex: corrosion)
Small grains properties
large boundary area
edge dislocation will meet a high angle grain boundary and will not form
colling metal quickly can create small grains
large grains properties
longer range crystal structure
edge dislocations easily propagate through low angle grain boundaries
how type and degree of defects effect material properties
point defects induce lattice strains and alter crystal strength
dislocations induce lattice strains and can move affecting ductility
grain size can alter material strength
porosity can affect bulk material strength