Fundamentals of Amines and Amides
1/20
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
21 Terms
What is the amine functional group
NH2
How to prepare an Amine from halogenoalkane
Heat
Excess NH3 (ethanolic)
What is the classifaction as primary, secondary or tertiary amines based on
based on the number of carbons bonded directly to the nitrogen.
Primary - 1 carbon
Secondary - 2 carbons
Tertiary - 3 carbon
what is aromatic amine.
If a benzene is directly bonded to the nitrogen in an amine group
what is an aliphatic amine.
if not benzene is directly bonded to the nitrogen in an amine group,
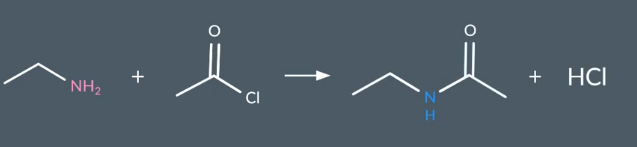
Ammine + Acyl Chloride
Amide
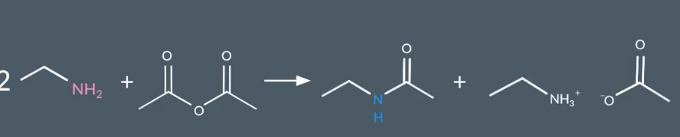
Ammine + Acid anhydride
Ammide
How ammides classifies
classify them based on the number of carbons bonded to the nitrogen.
Name ammine at end of chainf
root,ylamine
name tertiary ammine at end of chain
add approapate multiperler, root ylamine
name if ammine is not in the end of chain
root-an-position number-amine
lower prority group added to prefix as usual
what if ammine is not highest priorty
use prefix amino
name a primary amide
root-an - amide
naming a tertiary amide
group withot carbonyl group as subsituent
name of main chain add substient as pefix, with N before it, to show boned with nitrogen
name a teritary amide with identical substeints
add multiepr before perfix and inclued N,N before
name a tertiarty amide with diffrent substients
order substients in alphabetical order include N before each susbtient
what bond can Primary and secondary amines and amides form
can form hydrogen bonds to other primary and secondary amines and amides. This is because they have an N-H bond, with a lone pair on the nitrogen.
what do all amides and amines form with water
all amines and amides can form hydrogen bonds with water molecules
solubility of amines amides
all amines and amides can form hydrogen bonds with water molecules
This means that small amines and amides are highly soluble in water; their solubility decreases as the size of the molecule increases.
why can amines act as nucelophiles and electrophiles
because amines have a lone pair of electrons,