amount of substance & qualitative analysis
1/10
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
11 Terms
mole
number of atoms (unit measurement for amount of substancr)
avogadro’s constant
6.02 × 10²³ number of particles in each mole of carbon-12
amount of substance equation (n)
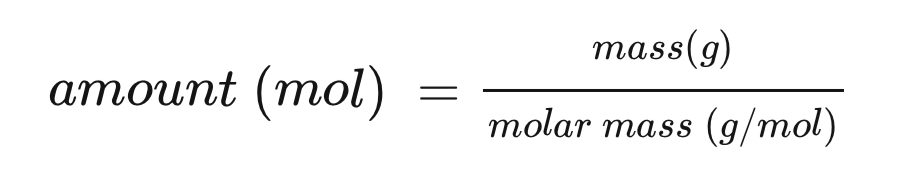
molecular formula
the number and type of atoms of each element in a molecule
empirical formula
the simplest whole number ratio of atoms of each element present in a compound
hydrated salt
ionic compound that contains water molecules chemically bound within its crystal lattice
water of crystallisation
water molecules that are part of the crystalline structure on component
anhydrous salt
salt contains no water of crystallisation
finding empirical formula from mass
1) moles = mass / Ar
2) divide each of these answers by smallest answer
3) change ratio into formula
changing empirical formula to molecular formula
1) Mr of actual molecule (told) / Mr of empirical formula = x
2) multiply x by formula
moles to atoms
moles x 6.02 × 10²³