Ch 5 Lecture 2 Respiratory Physio
1/23
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
24 Terms
what is the normal range for pH of the blood
normal blood pH 7.35-7.45
define volatile acids. give an example
an acid that can converted into a gas to be exhaled
ex: carbonic acid (H2CO3)
define nonvolatile acids. give examples
an acid that CANNOT be converted into a gas to be exhaled therefore it must be buffered by the KIDNEYS
ex: lactic acid, fatty acids, ketones
bicarbonate (HCO3-) is associated with the (lungs/kidneys) and (metabolic/respiratory) problems
bicarb is associated with the kidneys and metabolic problems
when looking at an acid or base problem, what is the first step?
look at the pH to determine if we are in acidosis or alkalosis
what is the cause of respiratory acidosis?
hypoventilation
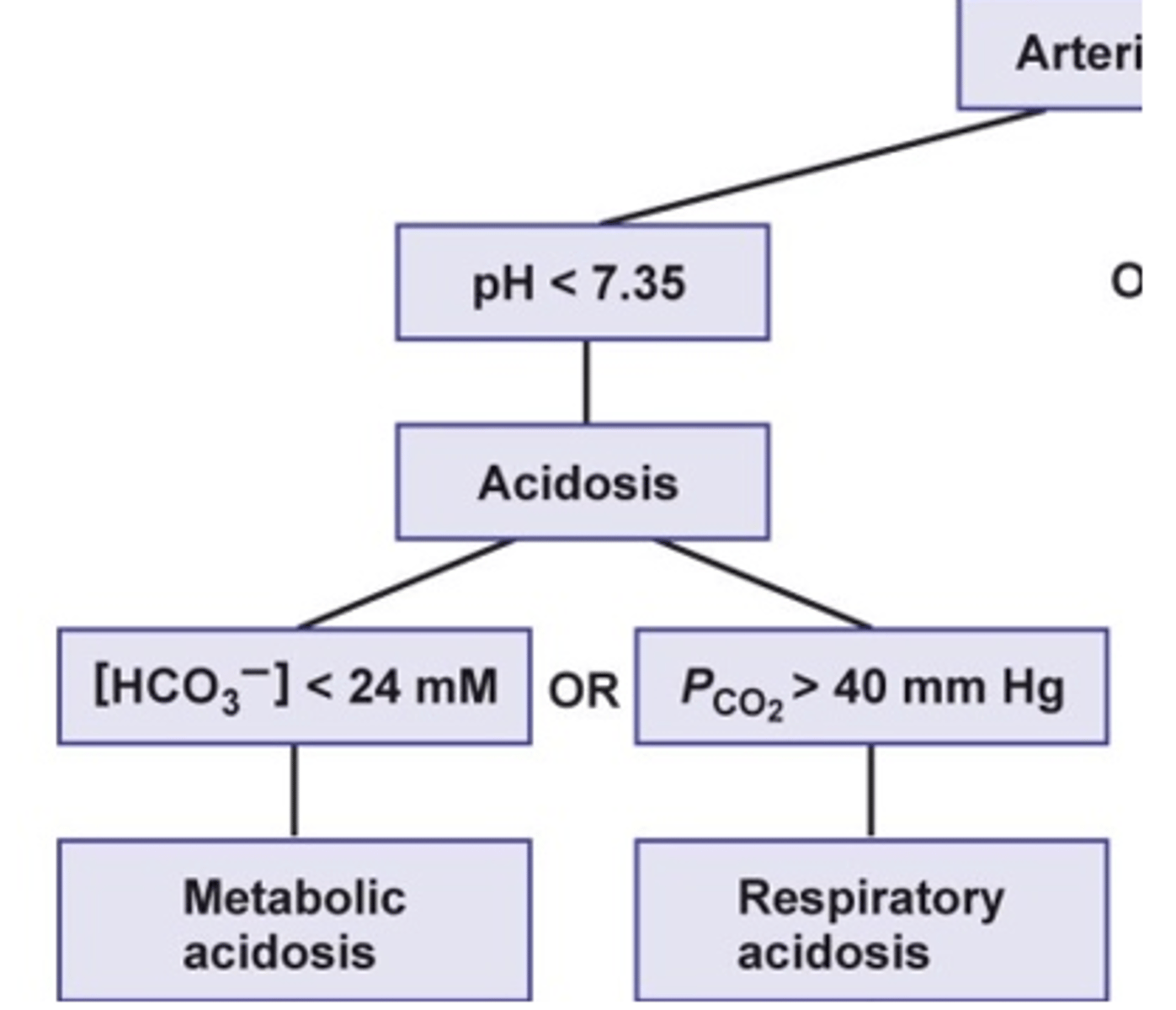
what happens to the CO2 and H+ levels in the blood during respiratory acidosis?
- increase CO2 = hypercapnia
- increase carbonic acid (H2CO3)
- increase H+ --> decrease pH
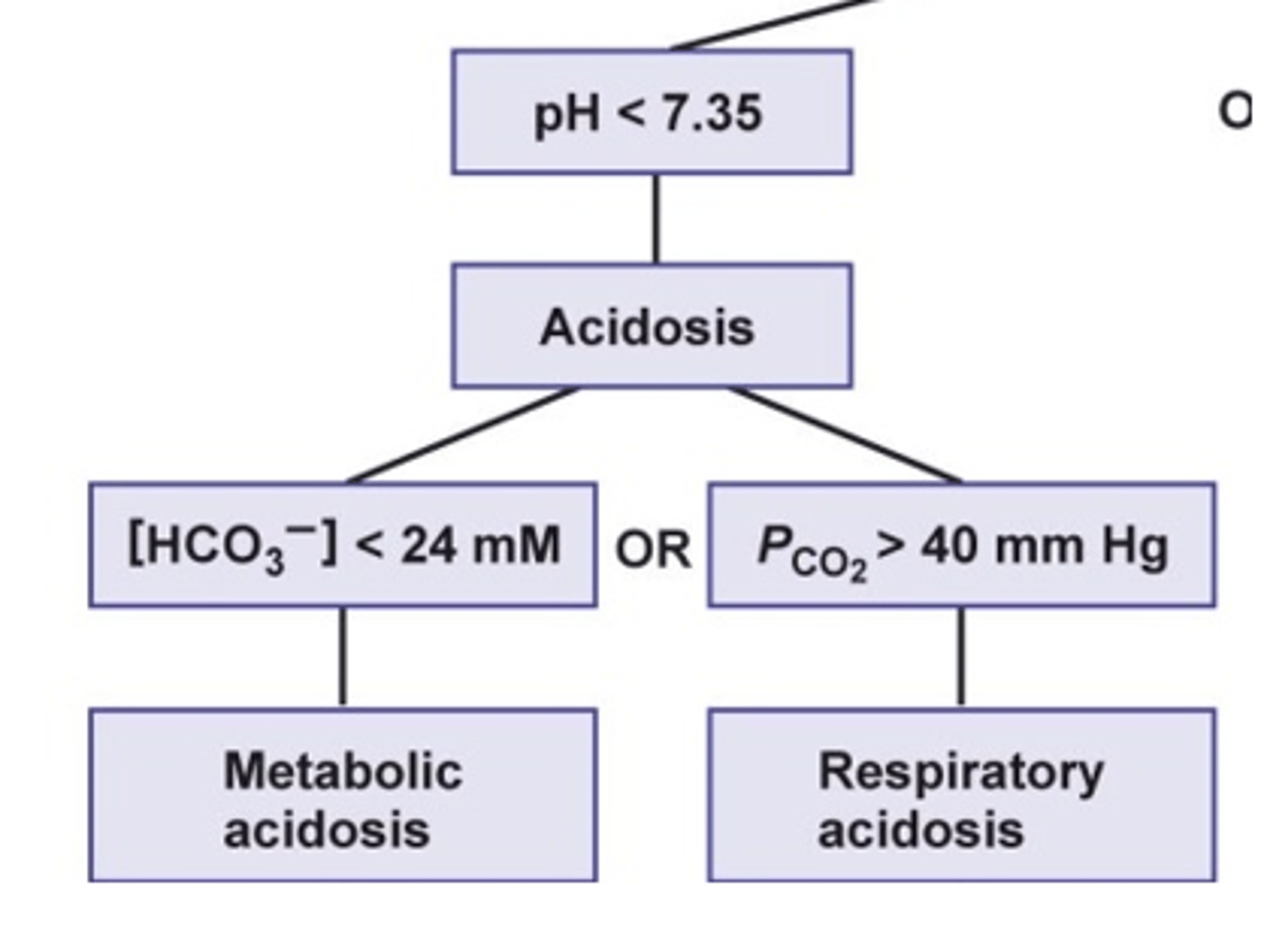
what are the causes of metabolic acidosis? (2)
- excessive production of acids
- prolonged diarrhea
- diarrhea: losing bicarbonate ion (HCO3-) via feces --> loss of negative charge through loss of bicarb --> no buffer for acids --> metabolic acidosis
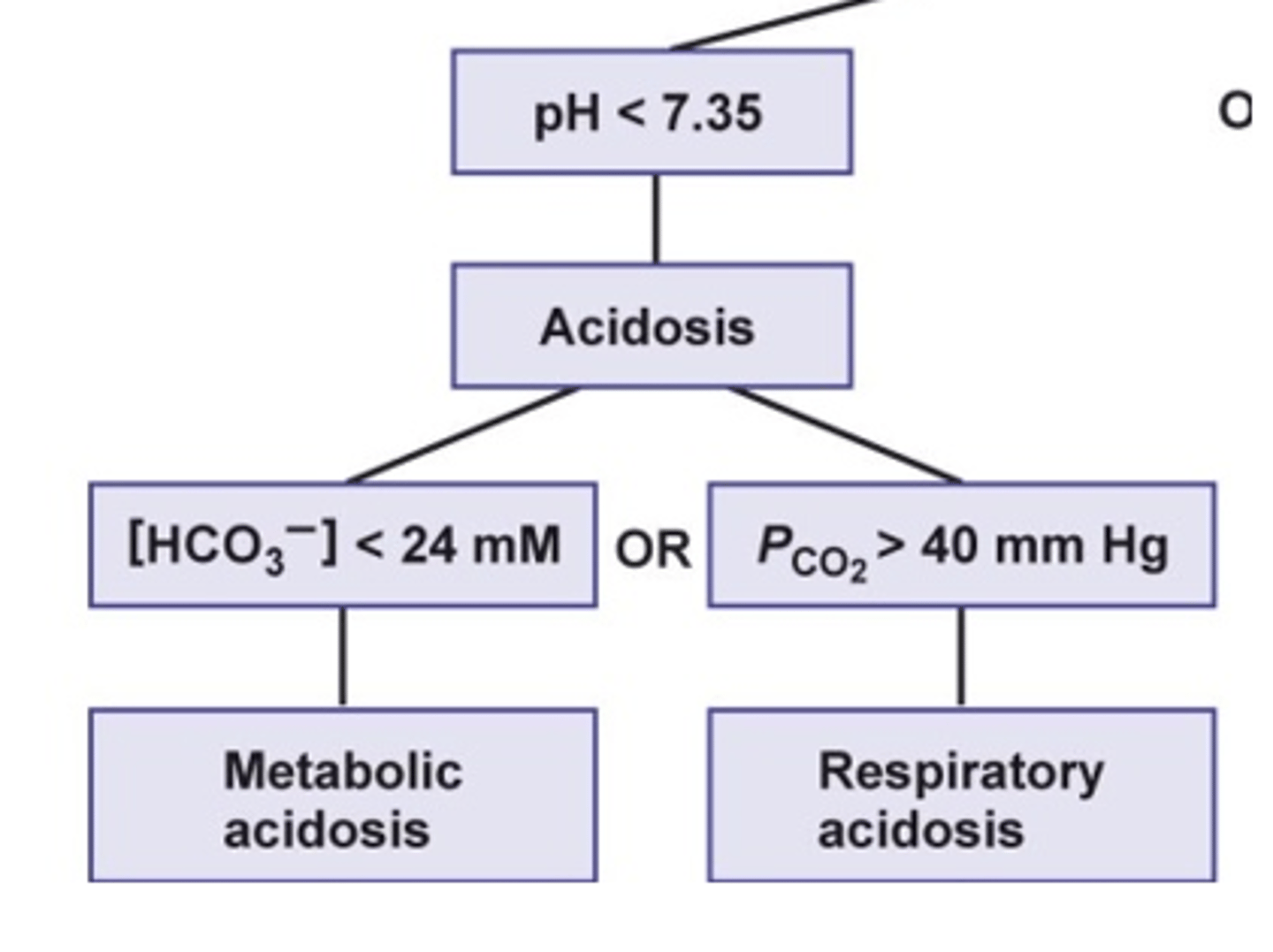
when looking at an acid or base problem, what is the second step?
look at the bicarbonate and CO2 levels to determine whether the issue is metabolic or respiratory
what is the cause of respiratory alkalosis?
hyperventilation
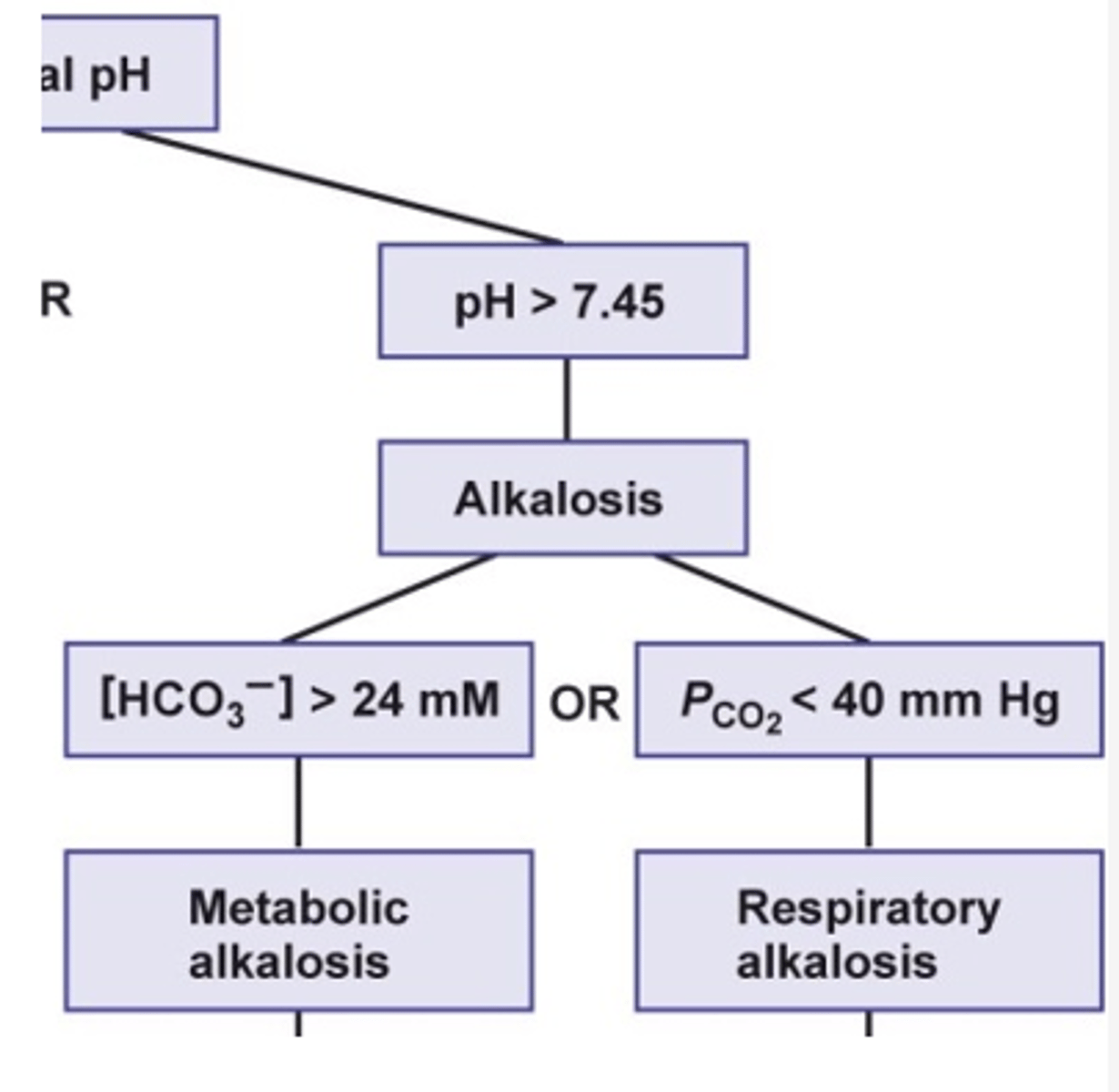
what happens to the CO2 and H+ concentration in respiratory alkalosis?
- decreased CO2 due to CO2 being "blown off"
- decrease formation of carbonic acid (H2CO3)
- decrease H+ --> pH increases --> alkalosis
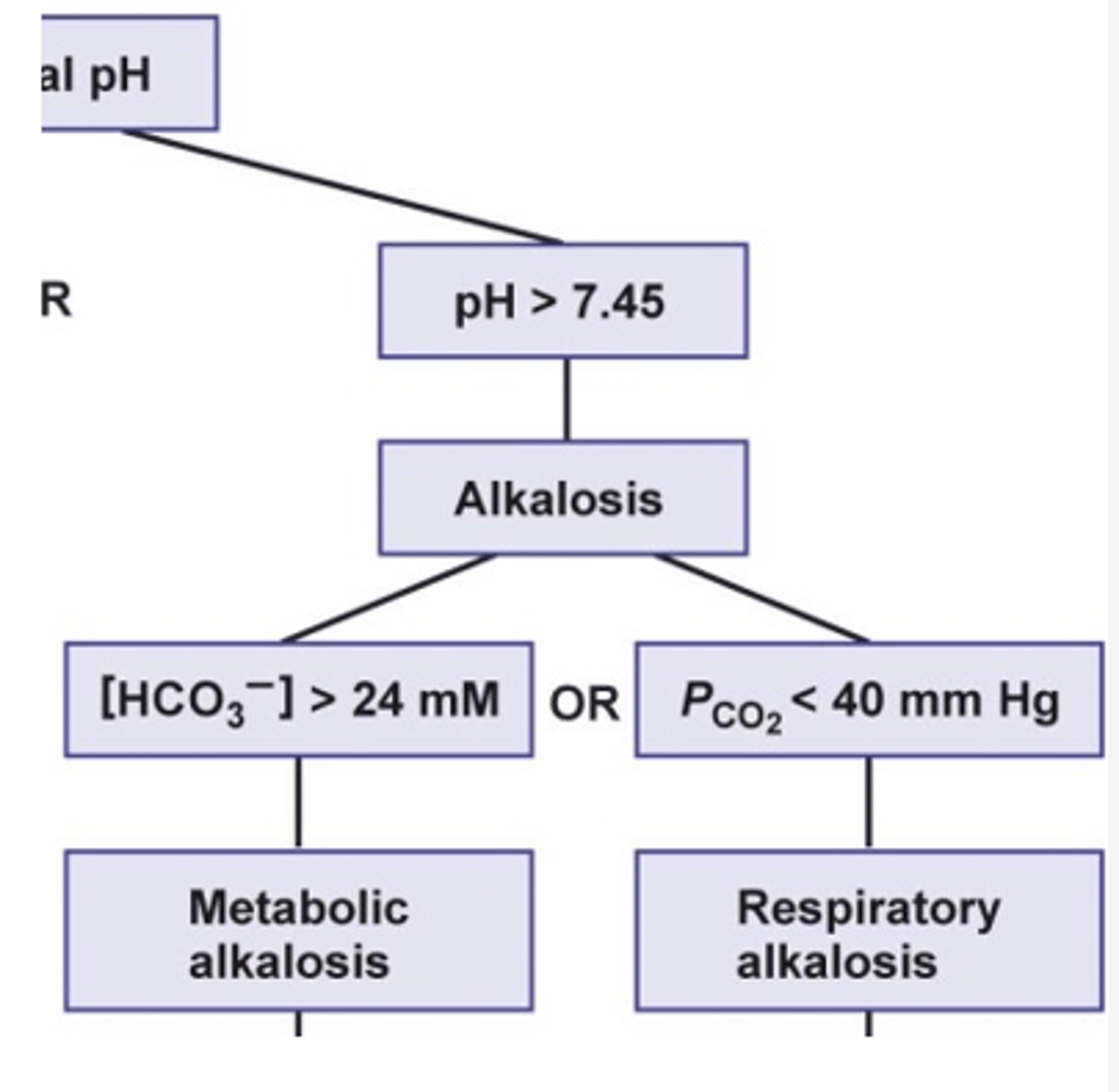
what is the cause of metabolic alkalosis?
- inadequate production of acids
- overproduction of bicarbonate
- loss of HCl via vomiting
- vomiting: HCl lost --> net loss of H+ lost --> increase pH --> alkalosis
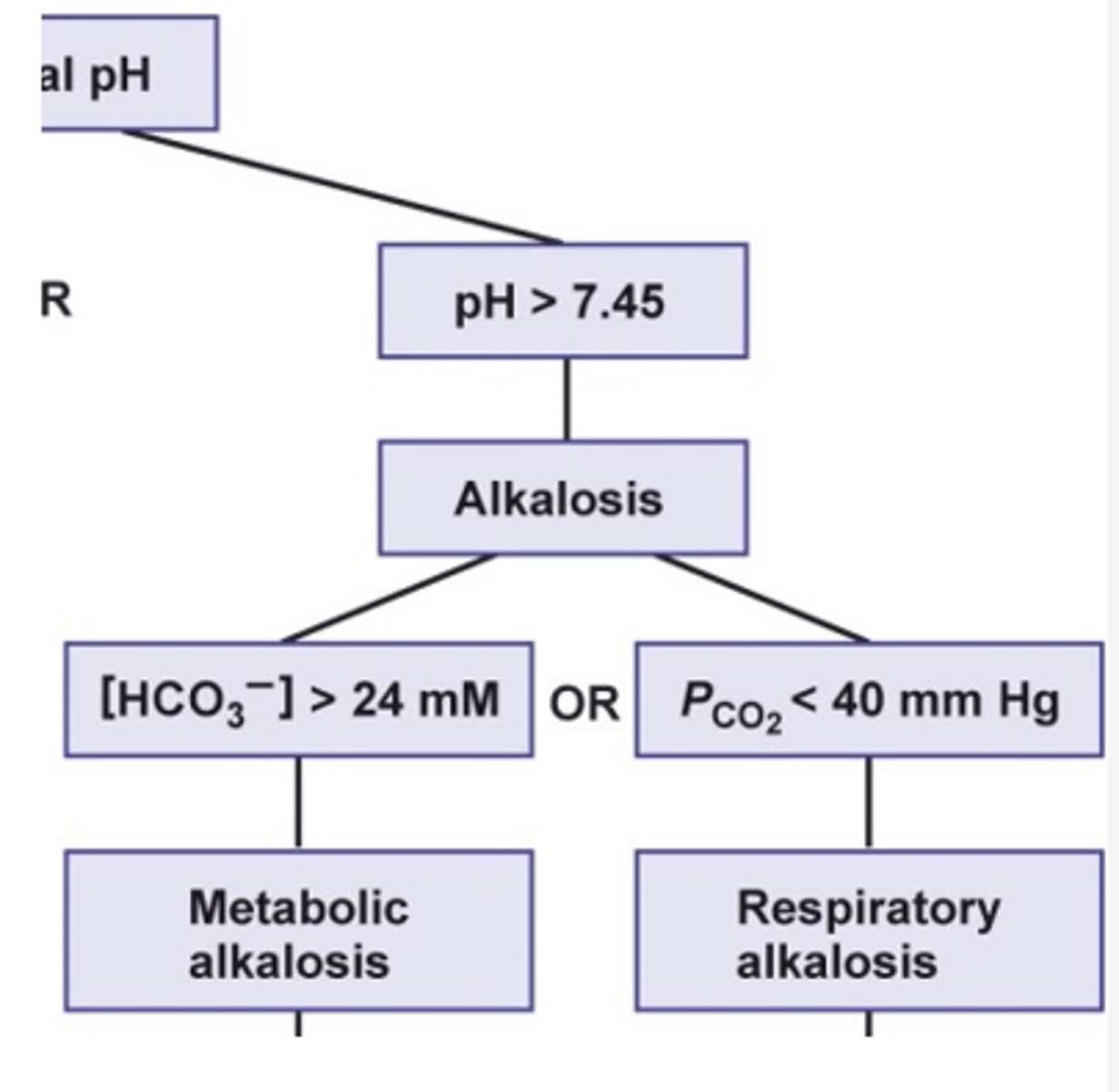
what happens to the bicarbonate concentrations in metabolic alkalosis?
- increase bicarbonate concentration
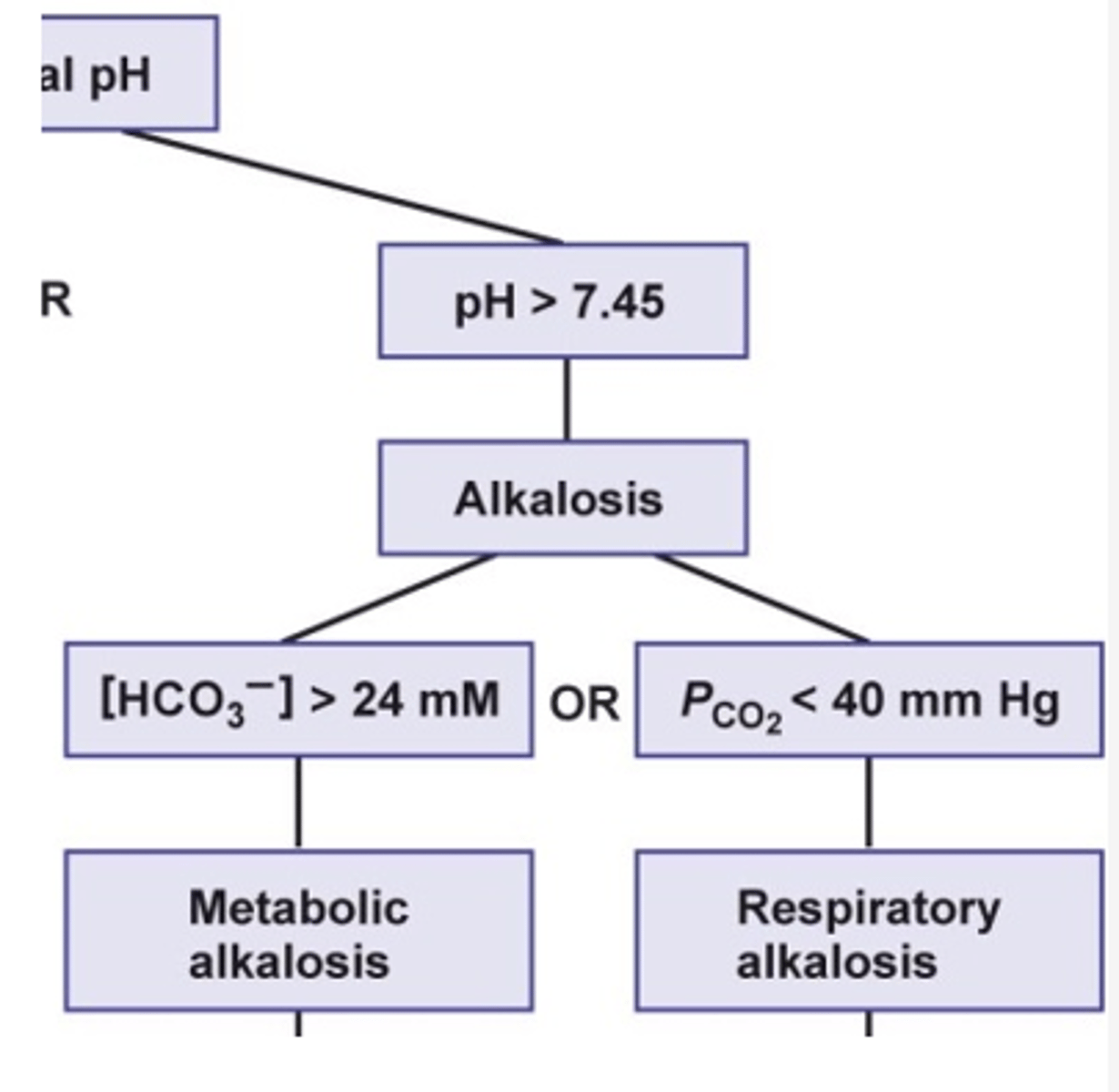
compensation: if patient is having a metabolic issue then they will compensate via the (metabolic/respiratory) system
compensation: metabolic issue --> compensate via the respiratory system
compensation: if patient is having a respiratory issue then they will compensate via the (metabolic/respiratory) system
compensation: respiratory issue --> compensate via the metabolic system
(T/F) there is more bicarb than CO2 in the blood because CO2 can cause acidosis or alkalosis
true
Exercise produces ______, _____ breathing to match _____ utilization and ____ production
Exercise produces faster, deeper breathing to match O2 utilization and CO2 production
at a light jog, explain what happens to these variables:
ventilation,
blood gases,
oxygen delivery to muscles,
oxygen extraction by muscles
ventilation- increased (need more O2)
blood gases- no change during light job
oxygen to muscles- increased
oxygen extracted by muscles- increases
At higher altitudes the percent of ____ in our body goes _____ because the _____ pressure of the atmosphere goes ____
At higher altitudes the percent of oxygen in our body goes down because the barometric pressure of the atmosphere goes down
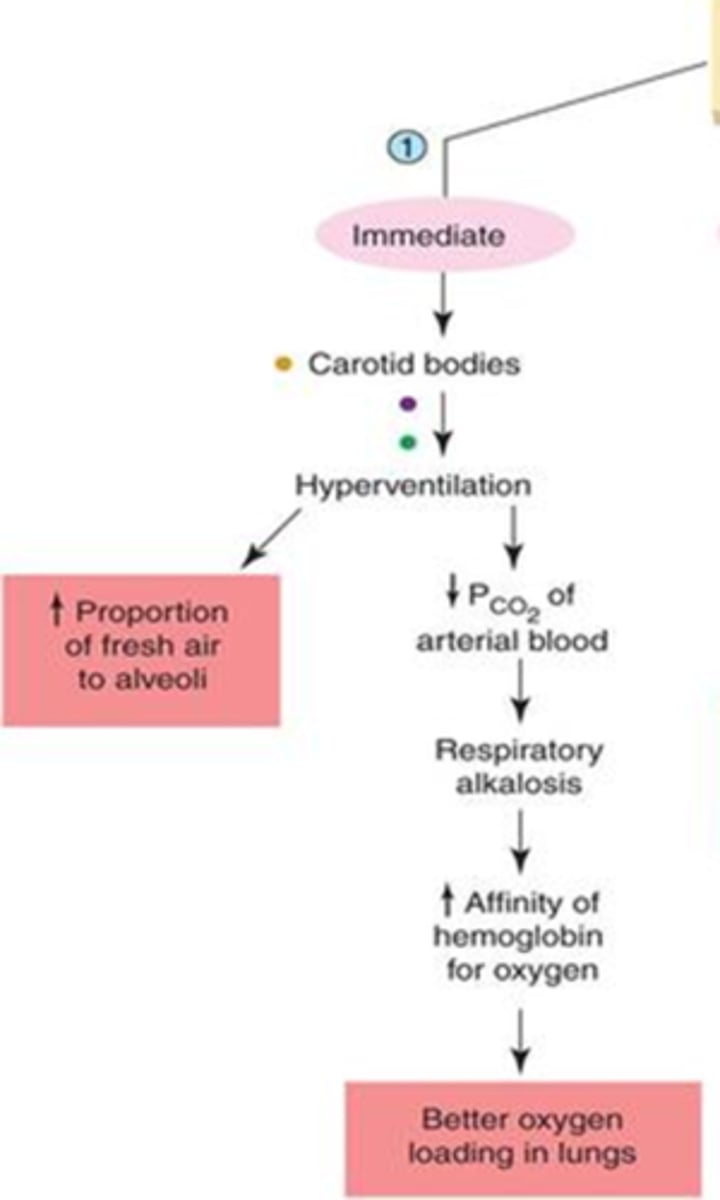
what is the body's immediate response to high altitude
1. decrease PO2 of atmosphere sensed by the carotid bodies
2. hyperventilation --> lowers CO2 -->
3. respiratory alkalosis achieved
5. increased affinity of Hgb for O due to alkalosis -->
6. better loading of oxygen in lungs
what is the body's response to high altitudes after a few days?
- increase in 2,3- DPG -->
- decreasing Hgb affinity for O
- better unloading of oxygen at tissues
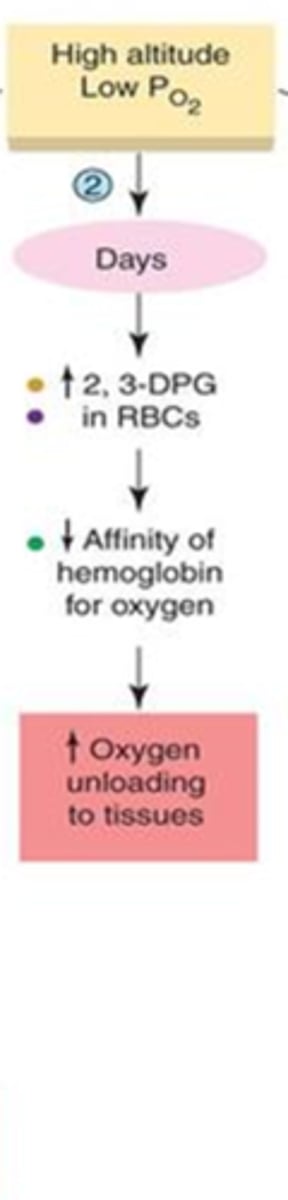
what is the body's final response to high altitudes?
- erythropoietin secretion by kidneys
- b/c hormones take a long time to be produced and secreted
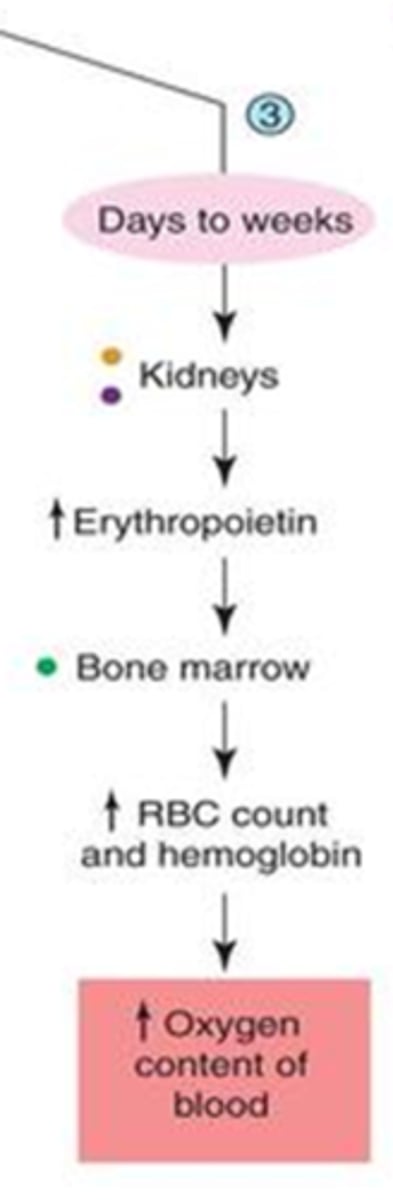
explain how secretion of erythropoietin increases oxygen within the blood?
1. erythropoietin secretion by kidneys -->
2. increase RBC production by bone marrow -->
3. increase O2 in blood
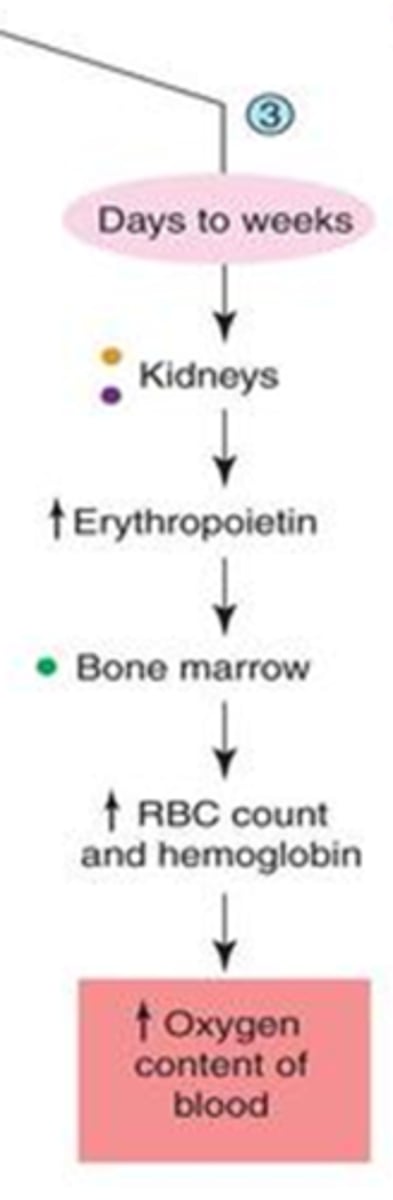
what do athletes do to increase O2 concentration in blood and therefore muscles during exercise?
- sleep in barometric chambers containing low O2 concentrations