bond enthalpies
1/3
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
4 Terms
what is average bond enthalpy?
energy required to break one mole of specified type of bond in gaseous molecule
bond enthalpies are always endothermic therefore they have positive enthalpy value
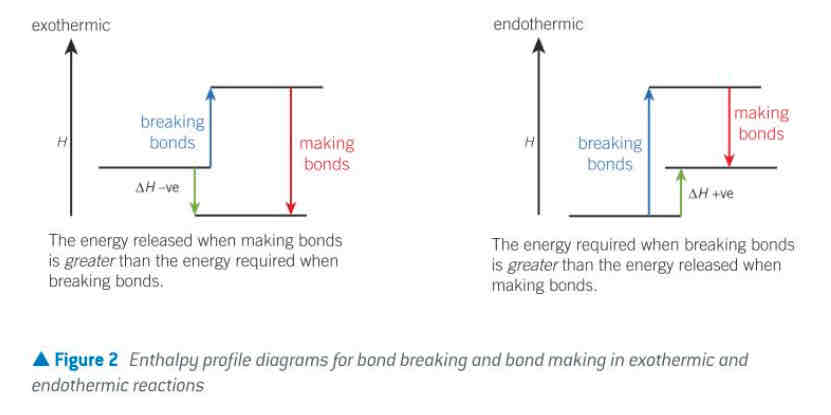
bond breaking and making…
endothermic: energy required to break bonds; delta H is positive
exothermic: energy released when bonds form; delta H is negative
calculating enthalpy changes from average bond enthalpies…
for a reaction involving gaseous molecules of covalent substances: reactants - products
actual energy involved in breaking/making individual bonds is slightly different

what is Hess’ Law?
states that if reaction takes place by two routes, and starting and finishing conditions are same, total enthalpy change is same for each route