C1.1: Enzymes & Metabolism | IB Biology HL
1/48
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
49 Terms
Define catalyst.
C1.1.1: Enzymes as catalysts.
A catalyst is a substance that speeds up the rate of a reaction without being permanently changed itself.
Define enzyme.
C1.1.1: Enzymes as catalysts.
A globular protein that acts as catalysts in metabolic reactions.
State the role of enzymes in the chemical reactions on which life is based.
C1.1.1: Enzymes as catalysts.
Enzymes speed up chemical reactions (metabolism). This is crucial, as without enzymes, metabolic reactions would occur very slowly at body temperature.
State that enzymes speed up chemical reactions without being altered.
C1.1.1: Enzymes as catalysts.
Enzymes speed up chemical reactions without being altered, so they can be reused.
Define metabolism.
C1.1.2: Role of enzymes in metabolism.
The complex network of interdependent and interacting chemical reactions occurring in living organisms.
Define specificity in relation to enzyme structure and function.
C1.1.2: Role of enzymes in metabolism.
Each enzyme has a specific structure that gives a specific function to catalyze one specific chemical reaction.
Specificity refers to an enzyme’s specific structure that allows it to fulfill a specific function (catalyze a specific reaction).
Outline how control of metabolism is regulated by enzymes.
C1.1.2: Role of enzymes in metabolism.
Metabolic pathways require different enzymes to catalyze each reaction. As a result, the cell controls metabolism through enzymes.
Having specific enzymes means the cell can control specific rates of reactions. There are mechanisms that speed up or slow down certain reactions depending on what the cell needs.
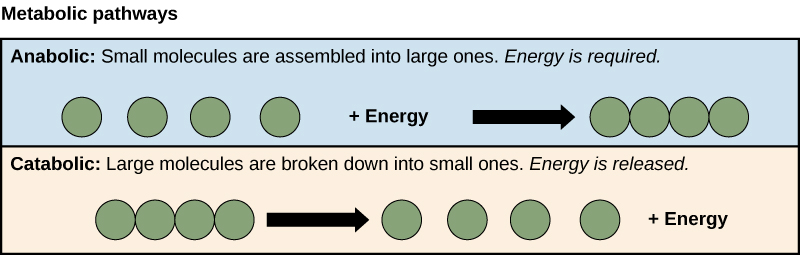
Contrast anabolic and catabolic reactions.
C1.1.3: Anabolic and catabolic reactions.
Anabolic reactions synthesize simpler molecules into a larger macromolecule. On the other hand, catabolic reactions break down complex molecules into smaller simpler molecules.
Anabolic reactions work through condensation reactions by using energy to create new bonds and producing water. Catabolic reactions work through hydrolysis reactions that use water to release bonds, producing energy.
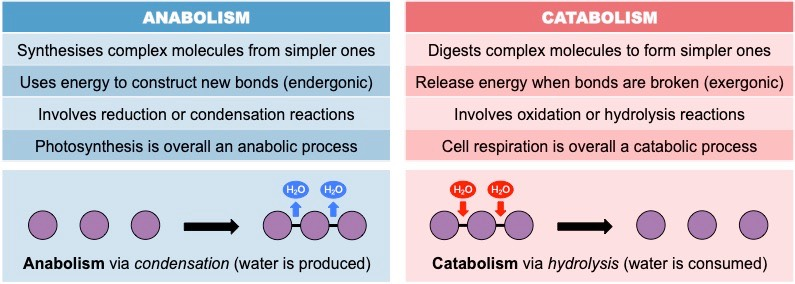
List three examples of anabolic processes.
C1.1.3: Anabolic and catabolic reactions.
Formation of macromolecules from monomers through condensation reactions
Protein synthesis
Glycogen formation
Photosynthesis
List examples of catabolic processes.
C1.1.3: Anabolic and catabolic reactions.
Hydrolysis of macromolecules into monomers
Digestion
Oxidation of substrates in respiration
Outline properties of globular proteins.
C1.1.4: Enzymes as globular proteins with an active site for catalysis.
Globular proteins are roughly circular. They have an irregular and wide range of R groups. Their functions are primarily physiological and play a functional role. They are generally soluble in water.
Examples of globular proteins include hemoglobin, insulin, immunoglobulin, and enzymes.
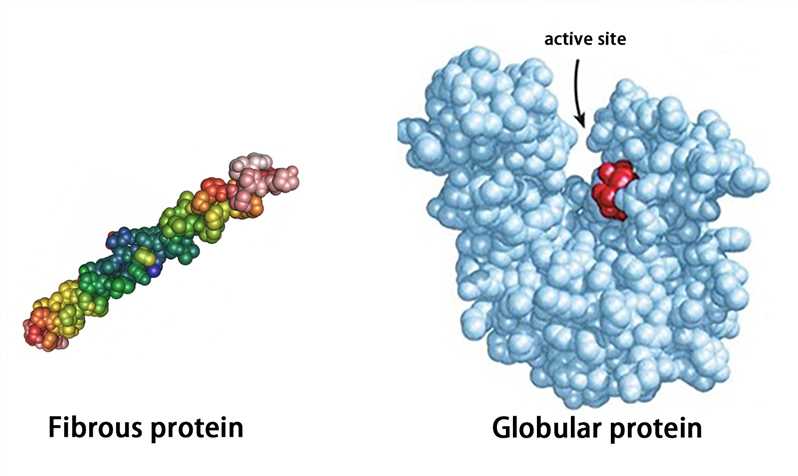
Define “active site.”
C1.1.4: Enzymes as globular proteins with an active site for catalysis.
The region where substrates bind and are catalysed into products
Explain the relationship between enzyme structure and enzyme specificity, including the structure and function of the active site.
C1.1.4: Enzymes as globular proteins with an active site for catalysis.
Substrates are reactants in enzyme-catalysed reactions. A substrate binds to an active site of an enzyme.
The active site is only composed of a few amino acids, but the 3D structure of the enzyme ensures that the active site can catalyse a specific reaction of the substrate. The active site’s complementary shape and chemistry allows one specific substrate to bind. Thus, the specific 3D structure allows the enzyme to complete one specific function.
Outline the stages of enzyme catalysis of a chemical reactions.
C1.1.5: Interactions between substrate and active site to allow induced-fit binding.
First, the active site and substrate come close together. The substrate enters the active site of the enzyme.
Then, an enzyme-substrate complex is formed so that the substrate fits into the active site (and only that active site because active sites are specific). The substrate induces the active site to change shape for an optimal fit.
Next, catalysis occurs at that active site. It provides the optimum conditions for the substrate to be converted into the product.
The product has different chemical properties than the substrate, so it is released from the active site. However, the enzyme’s structure remains the same so that it can be reused.
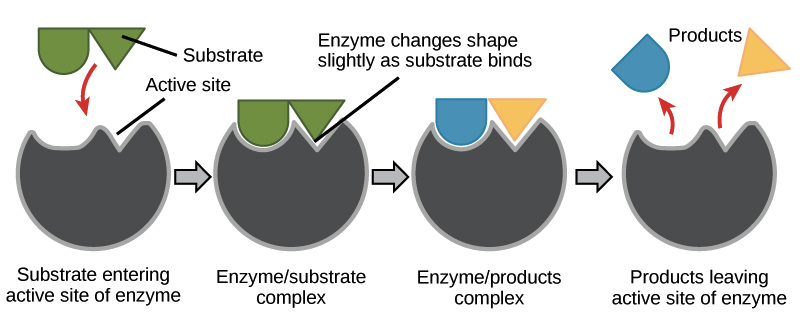
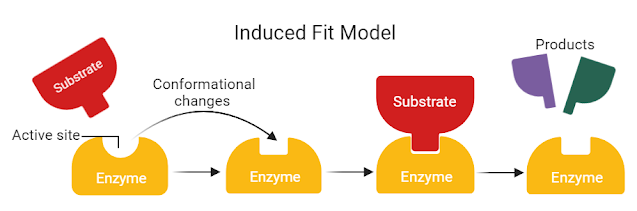
Describe the induced fit model of enzyme binding.
C1.1.5: Interactions between substrate and active site to allow induced-fit binding.
The induced fit model explains how substrates are converted into products by enzymes.
Namely, the substrate induces the active site of the enzyme to change shape so that there is an optimal fit between the substrate and the active site.
Unlike the lock and key model, which states that the enzyme does not change shape, the induced fit model explains that the shape of the active site does change because of the substrate.
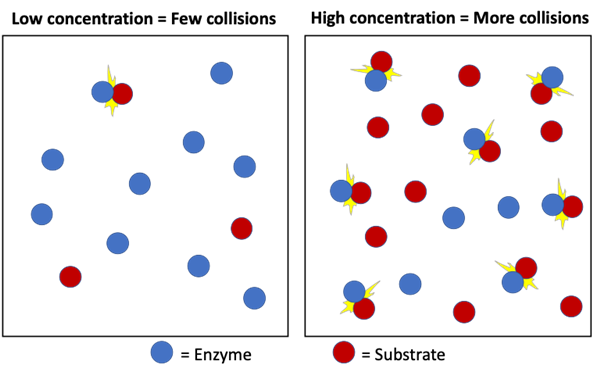
Explain the role of random collisions in the binding of the substrate with the enzyme active site.
C1.1.6: Role of molecular motion and substrate-active collisions in enzyme catalysis.
Random collisions are how substrates bind with the enzyme active site.
The particles need to randomly collide with the correct orientation with sufficient energy for them to bind and for the reaction to occur. The enzyme can only catalyze the reaction when the substrate randomly collides with it.
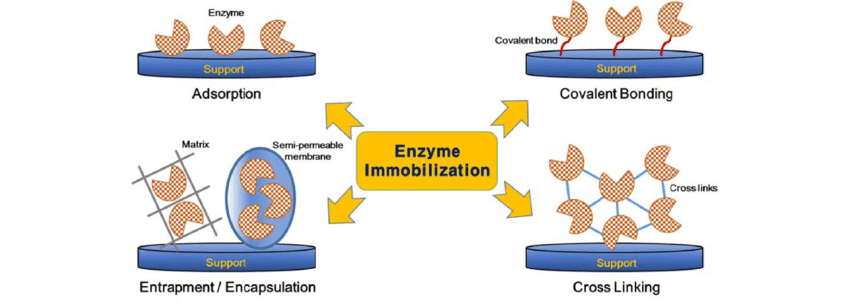
Outline ways to increase reaction rate in enzymes.
C1.1.6: Role of molecular motion and substrate-active collisions in enzyme catalysis.
The rate of enzyme-catalysed reactions increases when the number of collisions between substrates and active sites increases.
Increasing the temperature allows particles to move faster, so faster-moving substrates are more likely to randomly collide with an active site (and a reaction can occur).
Increasing the substrate concentration increases the chance of a substrate randomly colliding with an active site, which increases the rate of the reaction.
Immobilizing the substrate (particularly a large substrates like proteins are starch) can allow for repeated enzymatic hydrolysis of a macromolecule into monomers.
Immobilizing enzymes in membranes keeps the enzymes in close proximity to the substrate it catalyzes. The membrane maintains the stability of the enzyme.

Compare enzyme and substrate movement involved in reactions that occur in the cytoplasm with large substrates and with immobilized enzymes.
C1.1.6: Role of molecular motion and substrate-active collisions in enzyme catalysis.
Reactions with large substrates like proteins and starch can be immobilized. As a result, they can be repeatedly involved in reactions that break down a macromolecule into monomers.
Reactions with immobilized enzymes in membranes are held stable by the membrane. This keeps the enzyme in close proximity to the substrates it catalyzes.
Both increase the rate of reaction by immobilizing one of the reactants. However, in reactions with large substrates, the substrates are immobilized, while enzymes are immobilized when they are in the membrane.
Explain the relationships between the structure of the active site and enzyme-substrate specificity.
C1.1.7: Relationships between the structure of the active site, enzyme-substrate specificity and denaturation.
In enzyme action, the surface of a substrate makes contact with the active site of the enzyme. Then the enzyme and substrate change shape for an optimal fit. The enzyme-substrate complex temporarily forms. As a result, the activation energy is lowered, the existing atoms are rearranged, and the substrate is altered. The product (transformed substrate) is released from the activation site, and the unchanged enzyme can then combine with other substrate molecules.
The structure of the enzyme is key to its function. The active site must be a specific shape to form a specific enzyme-substrate complex.
Summarized by: E + S ↔ ES ↔ E + P
E is the enzyme
S is the substrate
ES is the enzyme-substrate complex
P is the product
Define denaturation.
C1.1.7: Relationships between the structure of the active site, enzyme-substrate specificity and denaturation.
The unfolding or breaking up of a protein that modifies its 3D structure.
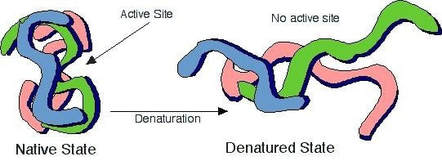
Outline the causes and effects of denaturation on enzyme structure and function.
C1.1.7: Relationships between the structure of the active site, enzyme-substrate specificity and denaturation.
Denaturation occurs when an enzyme is in an environment different from its optimal temperature and pH.
Being in such an environment causes the intramolecular bonds in the enzyme to be stressed and broken.
This causes the enzyme to denature, or to change in 3D shape. This change in shape results in a less effective enzyme because the active site also changes. Thus, the rate at which the enzyme works is affected.
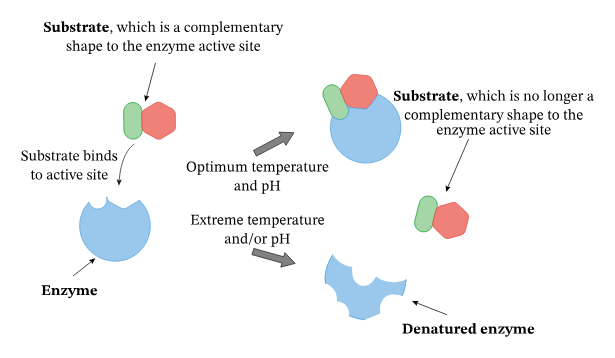
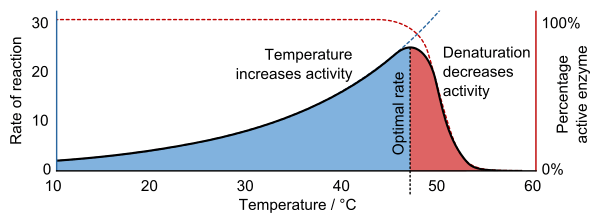
Explain the effects of temperature on enzyme structure and function with reference to collision theory, temporary and permanent denaturation.
C1.1.8: Effects of temperature, pH and substrate concentration on the rate of enzyme activity.
Higher temperatures increase the rate of reaction.
All chemical reactions are caused by molecules colliding. According to the Collision Theory, if molecules collide at a high enough speed and in the right way, a reaction may occur.
The rate of motion of enzymes and substrates depends on the temperature of the fluid it is in. If the temperature is higher, then there is greater kinetic energy, so there are faster moving molecules. Thus, molecules collide with each other more often and with greater energy. This increases the chance of a reaction occurring.
The effect of temperature is seen in solutions both with and without enzymes.
However, reactions with enzymes have an upper limit based on the temperature at which the enzyme denatures (3D shape is lost because intramolecular bonds are stressed or broken). This denaturation may be temporary because intramolecular bonds can reform when the enzyme returns to its optimal temperature. However, the denaturation may also be permanent if increasing the temperature prevents it from returning to its normal shape.
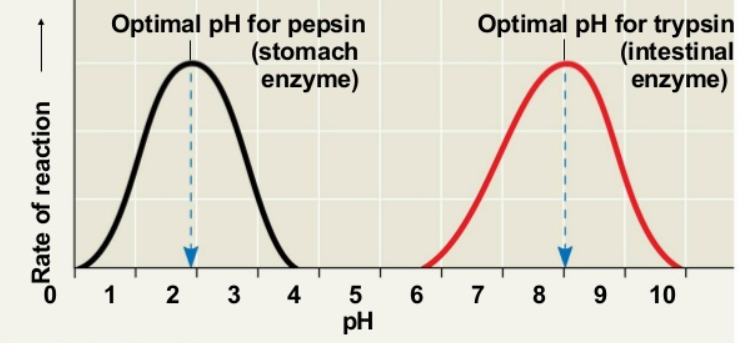
Explain the effects of pH on enzyme structure and function with reference to collision theory, temporary and permanent denaturation.
C1.1.8: Effects of temperature, pH and substrate concentration on the rate of enzyme activity.
Changes in pH can make an enzyme become less efficient.
The active sites of enzymes have amino acids that are either positively or negatively charged. The charged areas of the substrate must match the charged areas of amino acids in active sites.
If a solution becomes more acidic, then the concentration of hydrogen ions (H+) increases. These H+ ions may bond to the negative parts of the enzyme or structure, preventing the matching (like in the Collision Theory) between the enzyme in the substrate, limiting reactions. Similarly, if a solution becomes more basic, then the concentration of hydroxide ions (OH-) increases. The OH- ions may bond with the positive charge of the substrate or enzyme, preventing matching. As a result, the enzyme becomes less efficient.
If the pH drastically changes, the bonds within enzymes may break. Enzymes may lose shape and become denatured. Whether or not it is permanently denatured depends on whether covalent bonds (like peptide bonds) have been broken.
No one pH is best for all enzymes. Most enzymes in the human body do best in neutral environments. However, some enzymes like Pepsin (in the stomach) is more active in the acidic pH of the stomach.
Explain the effects of substrate concentration on enzyme structure and function with reference to collision theory, temporary and permanent denaturation.
C1.1.8: Effects of temperature, pH and substrate concentration on the rate of enzyme activity.
Increasing the substrate concentration increases the rate of reaction without changing the enzyme structure (denaturation).
According to the collision theory, reactions occur when the enzyme and substrate collide at the right energy level as well. Thus, if the concentration of reactant molecules (substrates) increase, then there are more molecules to react and collide with the enzymes.
However, enzymes have a maximum rate at which they can work because they only have one active site. Therefore, at a certain point adding more substrate will not increase the reaction rate if each enzyme molecule already has an occupied active site.
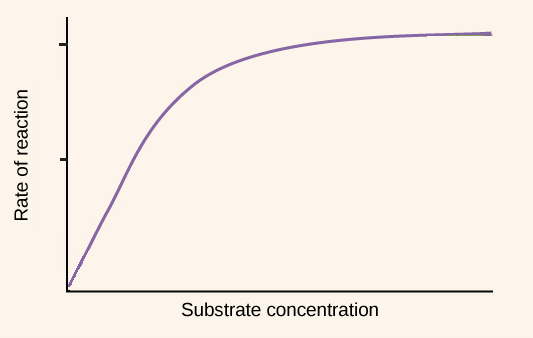
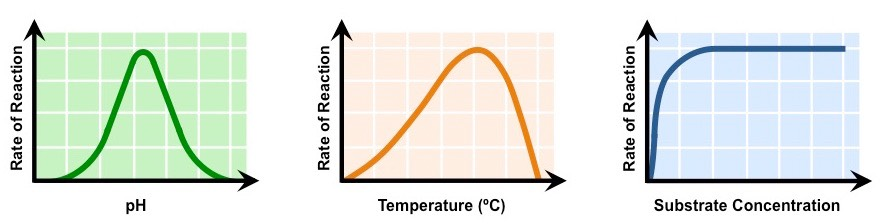
Draw and interpret graphs showing the effects of temperature, pH and substrate concentration of the activity of enzymes.
C1.1.8: Effects of temperature, pH and substrate concentration on the rate of enzyme activity.
Draw as in image shown.
The center of the pH graph (the highest point) is the optimal pH.
As the temperature increases, the rate of reaction increases up until a certain point where it begins decreasing.
As substrate concentration increases, the rate of reaction increases until it reaches a certain point where it remains constant.
A pH graph that is more towards the left (more acidic) performs better in more acidic conditions (like pepsin).
Define activation energy.
C1.1.10: Effect of enzymes on activation energy.
The minimum energy required to initiate a chemical reaction.
Activation energy is used to break or weaken the bonds in the substrate.
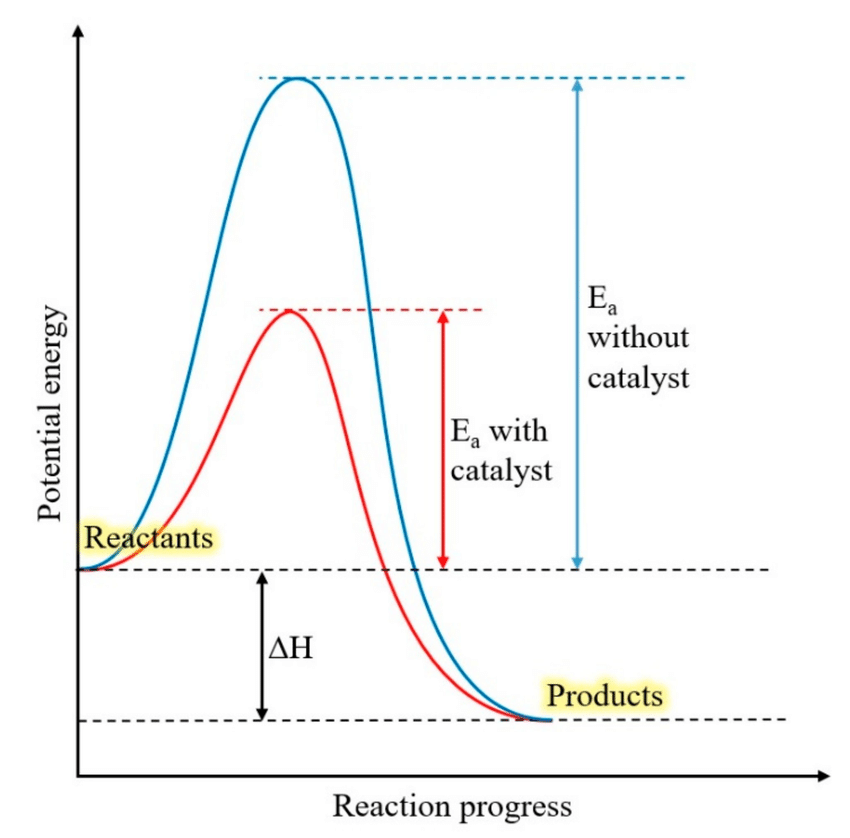
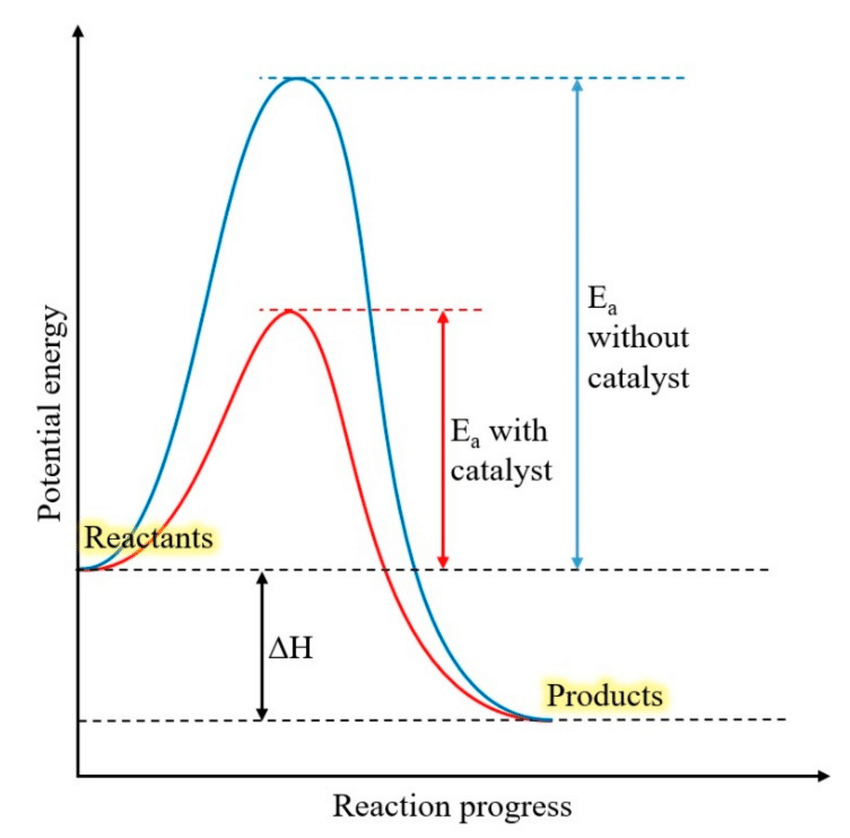
Explain the role of enzymes in lowering the activation energy of a reaction.
Interpret graphs showing the effect of lowering activation energy by enzymes.
C1.1.10: Effect of enzymes on activation energy.
Enzymes lower the activation energy barrier, which makes it easier for reactions to occur. Enzymes bind to reactant molecules so that the chemical bond-breaking and bond-forming processes take place more readily. This makes it easier for atoms to break and form new chemical bonds (speeding up the rate of reaction).
The graph shows how the activation energy required without a catalyst (the enzyme) is significantly higher than the activation energy required with a catalyst (an enzyme).
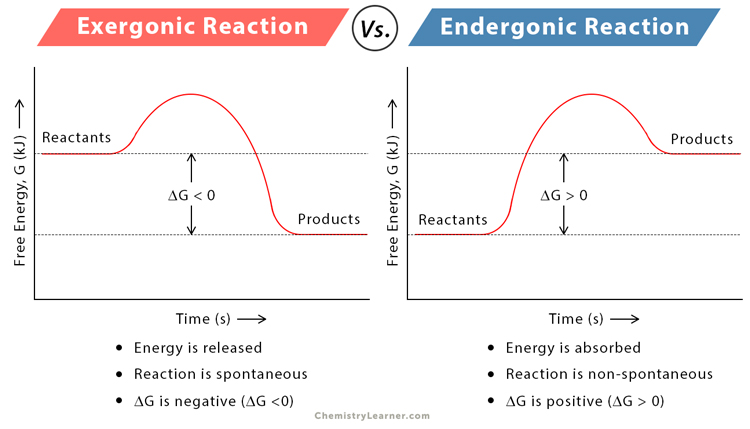
Distinguish exergonic and endergonic reactions (and provide examples).
C1.1.10: Effect of enzymes on activation energy.
Exergonic reactions release energy when they occur. However, endergonic reactions absorb energy when they occur.
The reactants of exergonic reactions have more energy than the products. The products of endergonic reactions have more energy than the reactants.
Exergonic reactions are typically catabolic reactions such as cellular respiration and the combustion of glucose. Endergonic reactions are typically anabolic reactions like photosynthesis.
Exergonic reactions are spontaneous (can release free energy), while endergonic reactions are non-spontaneous.
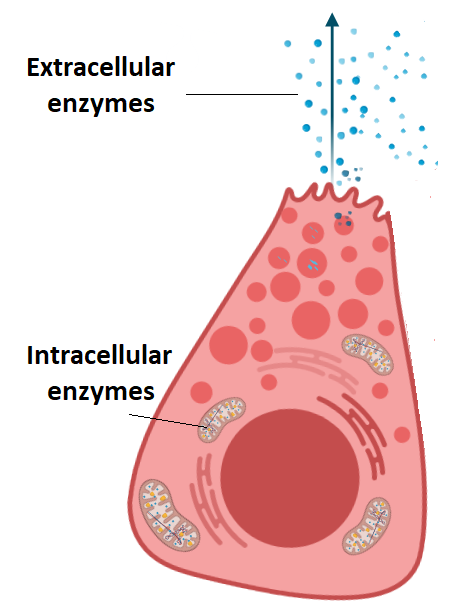
Compare intracellular and extracellular enzymes.
C1.1.11 (AHL): Intracellular and extracellular enzyme-catalyzed reactions.
Intracellular enzymes work within the cells where they are made, while extracellular enzymes work outside of cells.
Intracellular enzymes are synthesized by free ribosomes in the cytoplasm, while extracellular enzymes are synthesized by ribosomes on the rough endoplasmic reticulum (ER) and then sent to the Golgi apparatus to be packaged into secretory vesicles and then released by exocytosis.
Intracellular enzymes help with metabolic processes such as glycolysis and the Krebs cycle. Extracellular enzymes help with chemical digestion (such as amylase in saliva or lactase breaking down lactose).
State an example of an intracellular metabolic reaction and an extracellular metabolic reaction.
C1.1.11 (AHL): Intracellular and extracellular enzyme-catalyzed reactions.
Examples of an intracellular metabolic reaction is glycolysis and the Krebs cycle.
An example of an extracellular metabolic reaction is chemical digestion.
Outline the generation of heat energy by the reactions of metabolism.
C1.1.12 (AHL): Generation of heat energy by the reactions of metabolism.
Because metabolic reactions are not 100% efficient in energy transfer, heat is an inevitable natural byproduct of metabolism. In other words, some of the chemical energy is transferred into heat/thermal energy.
Describe the importance of heat production from metabolic reactions.
C1.1.12 (AHL): Generation of heat energy by the reactions of metabolism.
Metabolic reactions produce heat, which is important for birds, mammals, and some other animals. Endotherms are animals that maintain a constant body temperature, and such animals use the heat from metabolism to maintain a constant body temperature. They use the heat from metabolism to maintain a stable internal temperature.
State the reason for metabolic pathways.
C1.1.13 (AHL): Cyclical and linear pathways in metabolism.
Metabolic pathways transform matter and energy.
Compare linear metabolic pathways with cyclical reaction pathways.
C1.1.13 (AHL): Cyclical and linear pathways in metabolism.
Linear metabolic pathways are a step-by-step sequence where the product of one reaction becomes the substrate for the next. It produces a final product through a series of metabolic reactions.
On the other hand, cyclical metabolic pathways are a cycle where the starting reactant is produced at the end (unlike in linear, where the final product is different from the reactant).
However, both linear and cyclical reaction pathways use the product of one reaction as the substrate/reactant for the next reaction. Each chemical reaction requires specific enzymes.
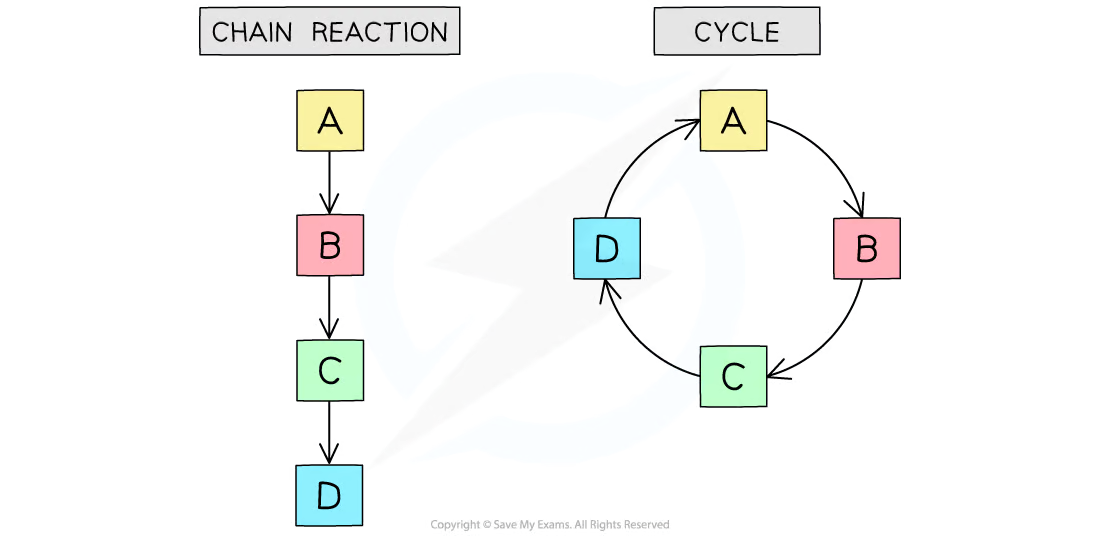
State an example of a linear metabolic pathway and a cyclic metabolic pathway.
C1.1.13 (AHL): Cyclical and linear pathways in metabolism.
Glycolysis is an example of a linear metabolic pathway. The initial reactant is glucose, and a series of reactions lead the products to continually be reacted on until it produces ATP and pyruvate.
The Krebs cycle in respiration and the Calvin Cycle in photosynthesis are examples of cyclical pathways.
Define enzyme inhibitor.
C1.1.14 (AHL): Allosteric sites and non-competitive inhibition.
Enzyme inhibitors are chemicals that bind to the enzyme and stop or slow down the activity of an enzyme.
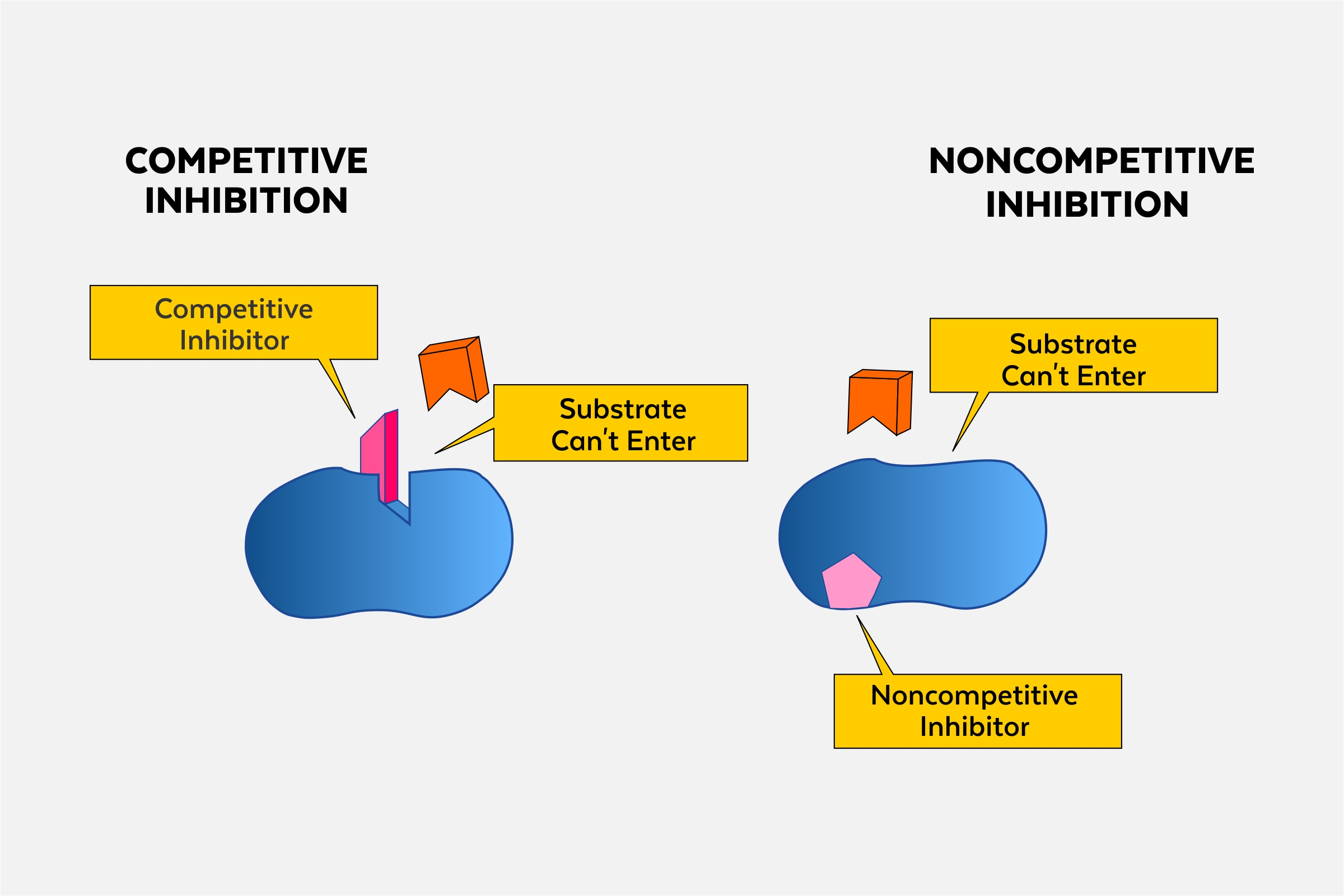
Outline the structure and function of an allosteric site.
C1.1.14 (AHL): Allosteric sites and non-competitive inhibition.
Allosteric sites are any place where the inhibitor binds to that is not the active site (non-competitive). It may be a different location depending on the enzyme.
The allosteric site has a specific structure that the inhibitor binds to.
The purpose of the allosteric site is to provide a place for the inhibitor to bind. As a result, the binding causes the shape of the active site to change so that the substrate can no longer bind to the enzyme.
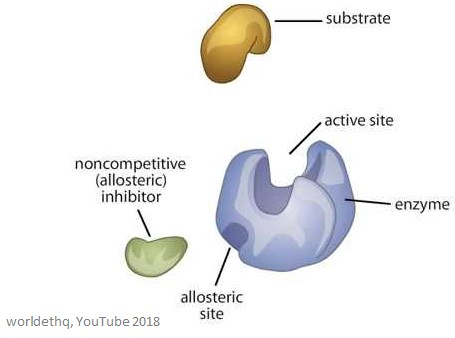
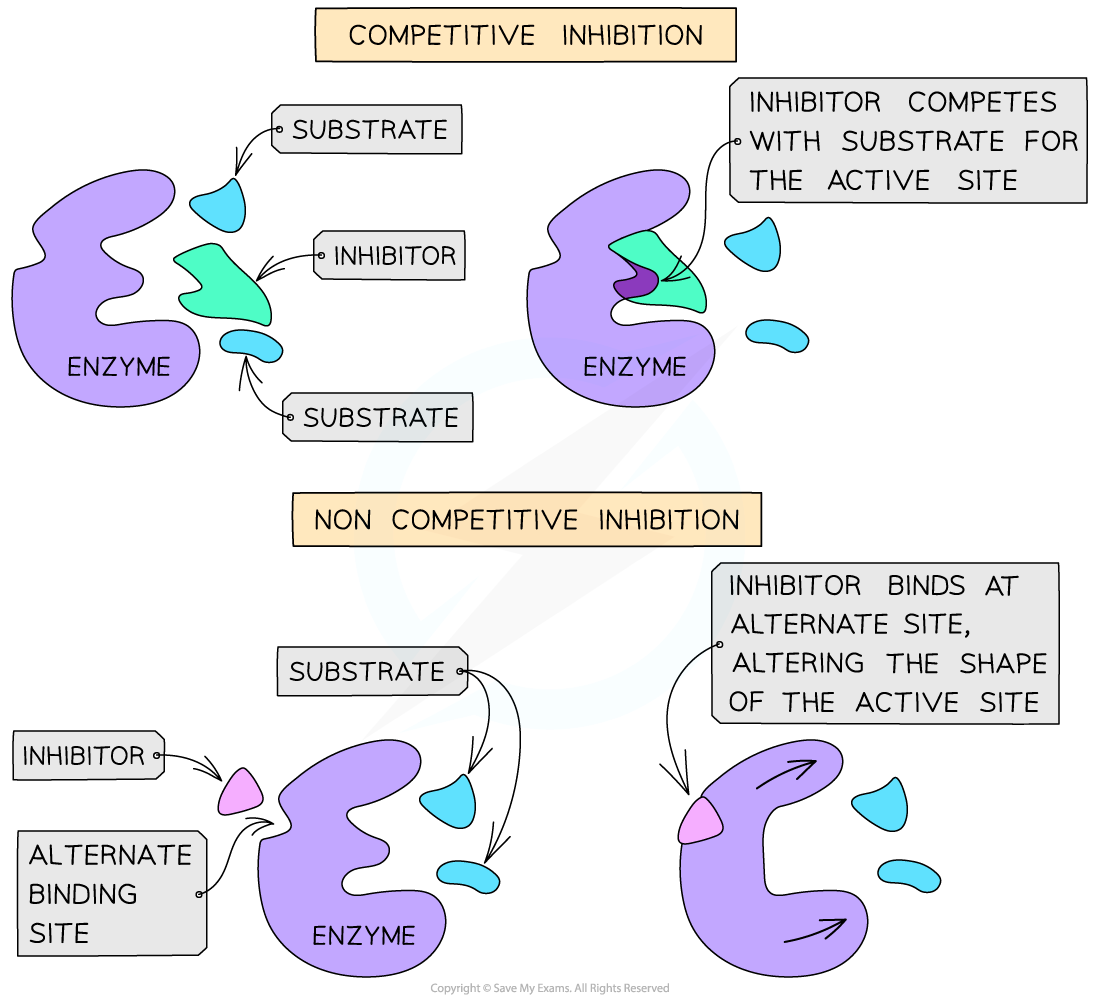
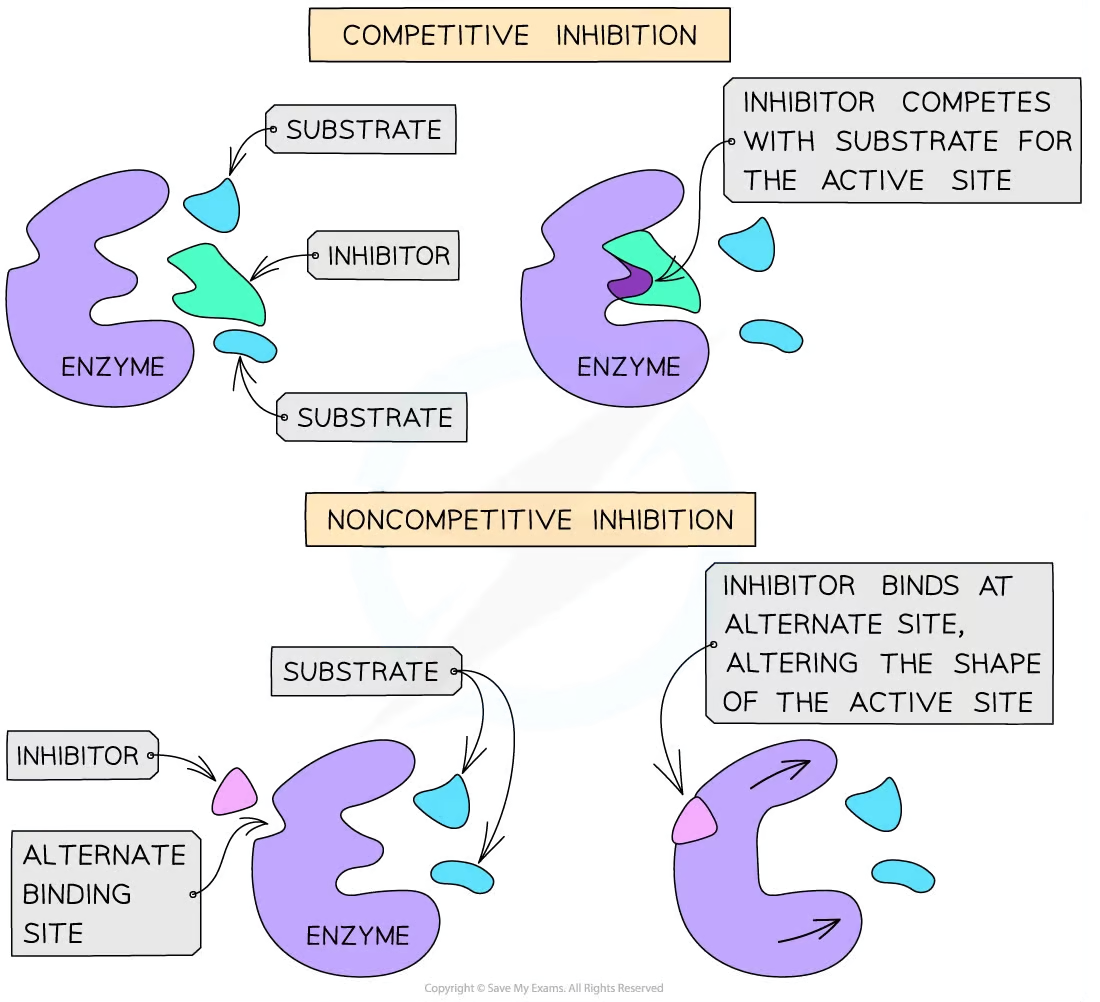
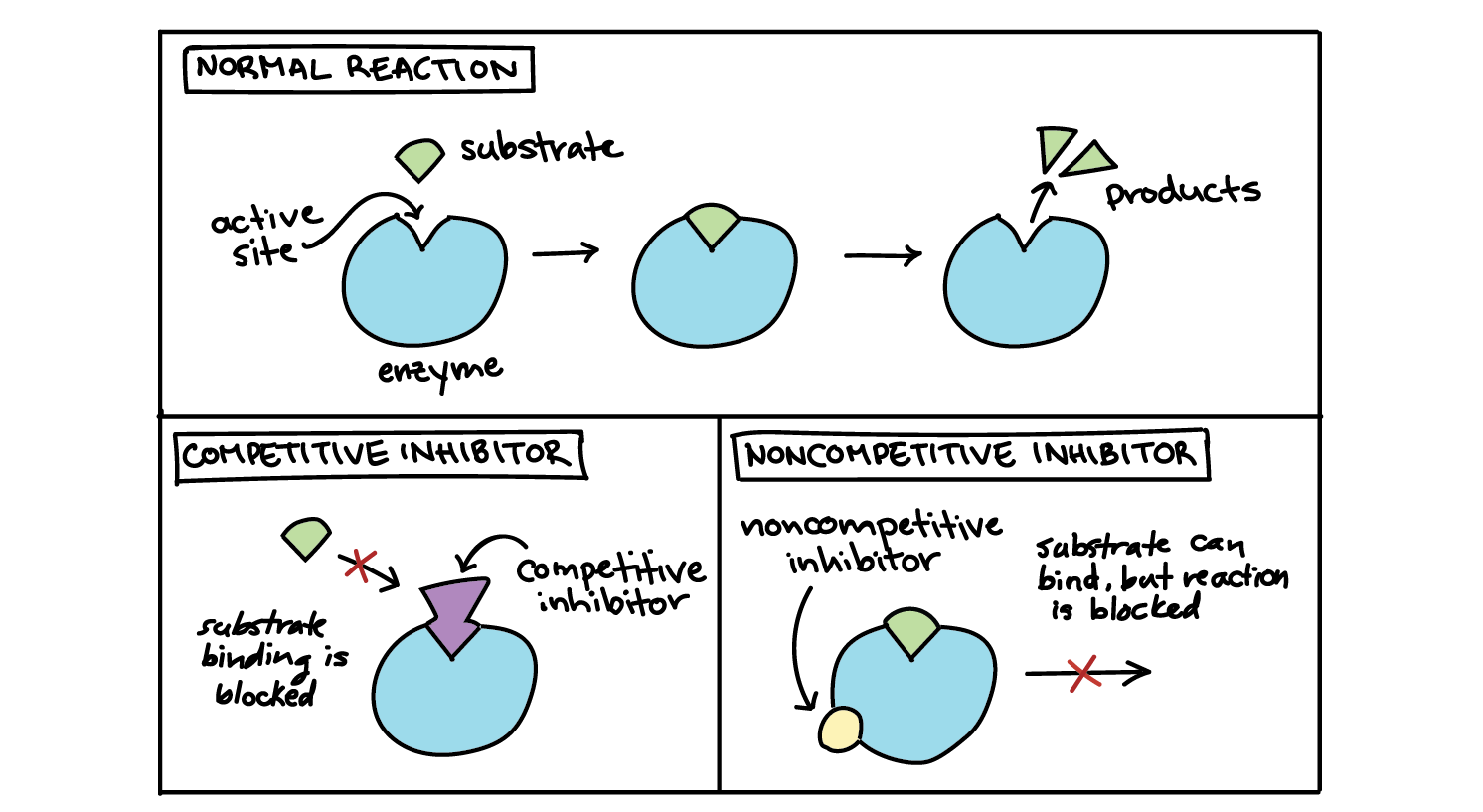
Describe the mechanism of action of non-competitive enzyme inhibitors.
C1.1.14 (AHL): Allosteric sites and non-competitive inhibition.
Non-competitive enzyme inhibitors are different in structure and chemistry to the substrate. Thus, they bind to the allosteric site on an enzyme, not the active site. As a result, this changes the structure of the active site, preventing the substrate from binding. This reduces the number of functioning enzymes, but the binding of the inhibitor to the allosteric site is reversible.
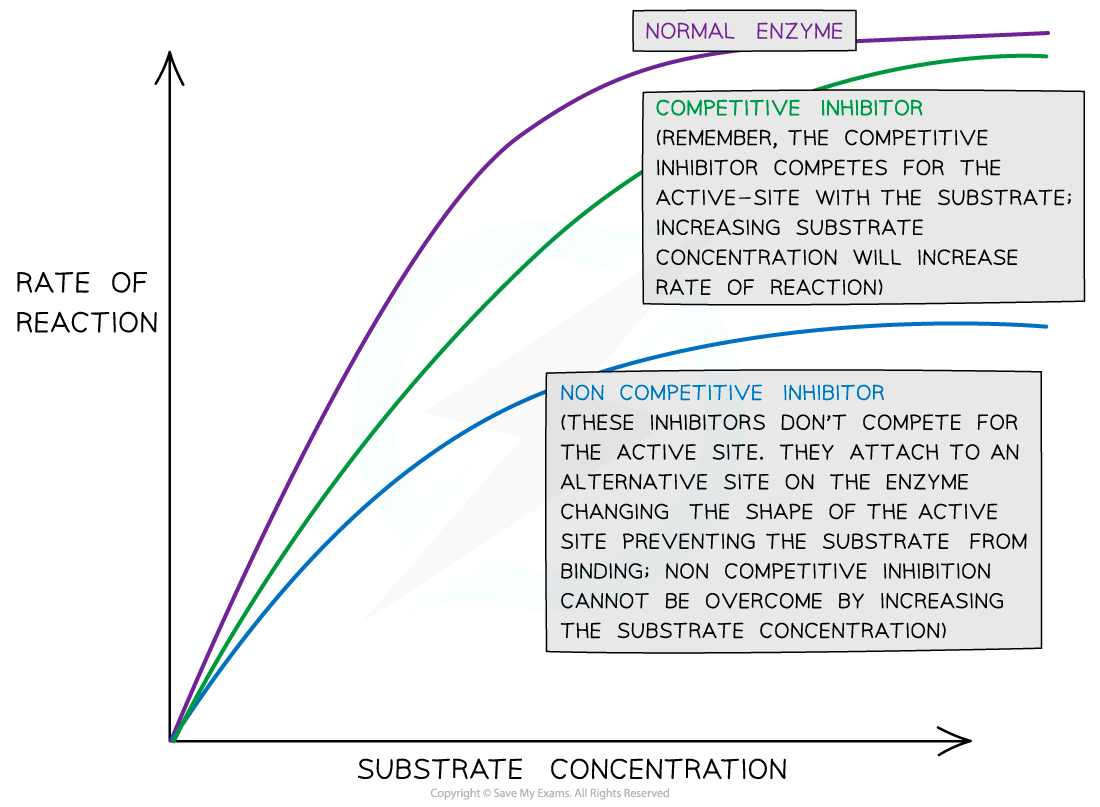
Describe how substrate concentration impacts the rate of reaction for a solution with non-competitive inhibitors.
C1.1.14 (AHL): Allosteric sites and non-competitive inhibition.
Non-competitive inhibitors reduce the number of functioning enzymes because enzymes with the inhibitor cannot catalyze the substrate.
However, increasing the substrate concentration will still increase the rate of reaction. More substrate molecules will collide with the active sites of enzymes that are not inhibited, but this is only for the enzymes that are not inhibited.
Describe the mechanism of action of competitive enzyme inhibitors.
C1.1.15 (AHL): Competitive inhibition as a consequence of an inhibitor binding reversibly to an active site.
Competitive enzyme inhibitors have a similar shape and chemistry to the substrate. They can also bind to the active site of the enzyme, which prevents the substrate from entering the active site. Thus, the competitive inhibitors compete with the substrates to enter the enzyme’s active site
Outline the functions of statins as an example of a competitive inhibitor.
C1.1.15 (AHL): Competitive inhibition as a consequence of an inhibitor binding reversibly to an active site.
Statins are a drug that lower cholesterol. They are competitive inhibitors that competes for the active site of the enzyme that produces cholesterol in the liver. This, in turn, allows statins to lower cholesterol production.
Describe how substrate concentration impacts the rate of reaction for a solution with competitive inhibitors.
C1.1.15 (AHL): Competitive inhibition as a consequence of an inhibitor binding reversibly to an active site.
When an inhibitor is bound, the substrate cannot enter, so the enzyme cannot catalyze the reaction. This reduces enzyme activity because fewer substrates can bind, and there is a slower reaction rate.
However, increasing the substrate concentration can boost enzyme activity because it leads to more active enzymes and fewer inhibitors that can bind. The higher substrate concentration increases the likelihood of substrates entering active sites and the reduced chance for inhibitors to bind.
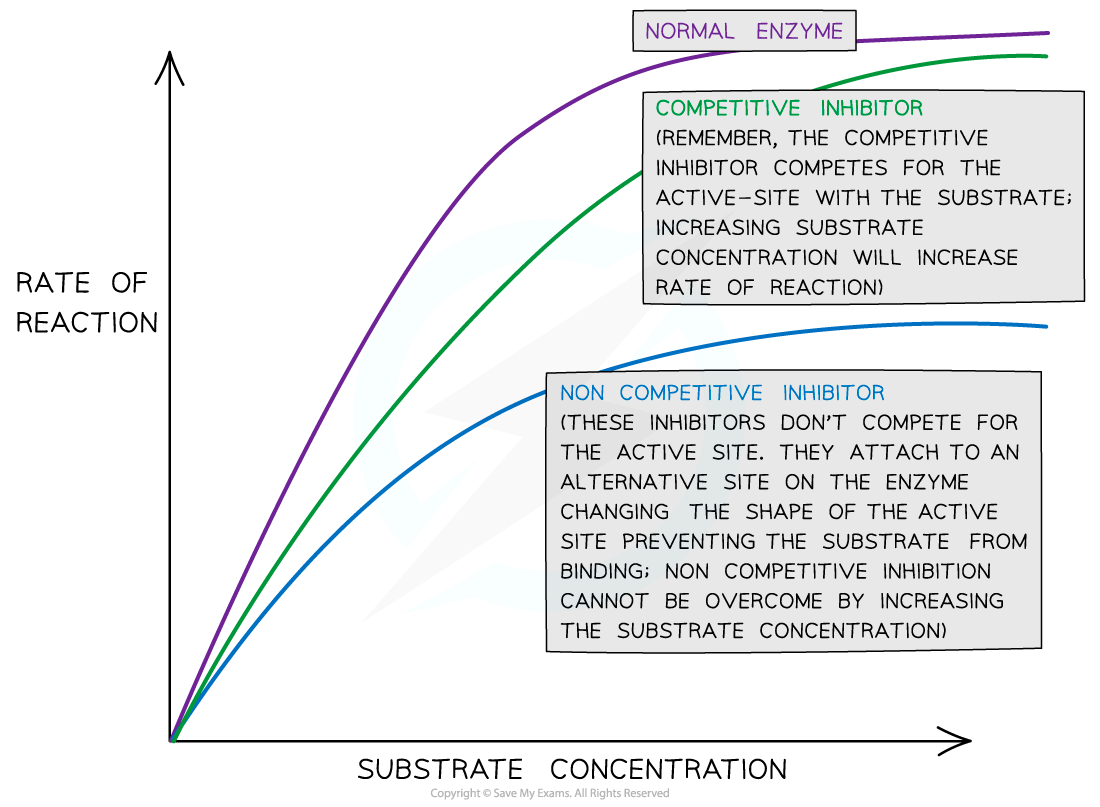
Explain why the rate of reaction with increasing substrate concentration is lower with a non-competitive inhibitor compared to a competitive inhibitor.
C1.1.15 (AHL): Competitive inhibition as a consequence of an inhibitor binding reversibly to an active site.
Non-competitive inhibitors do not compete with the substrate to bind to the enzyme; instead, it binds to the allosteric site to change the shape of the active site. Therefore, increasing substrate concentration will not impact whether or not the non-competitive inhibitor binds to the allosteric site and prevents the substrate from binding to the active site for a reaction.
Conversely, competitive inhibitors do compete with the substrate to bind to the enzyme’s active site. Increasing substrate concentration decreases the chance for the inhibitor to inhibit the reaction, so there is a greater effect on reaction rate.
Although increasing substrate concentration for non-competitive inhibitors does increase the rate of reaction for the enzymes that are not inhibited, it does not have as large of an effect than for competitive inhibitors because the non-competitive inhibitors themselves are not impacted by the substrates.
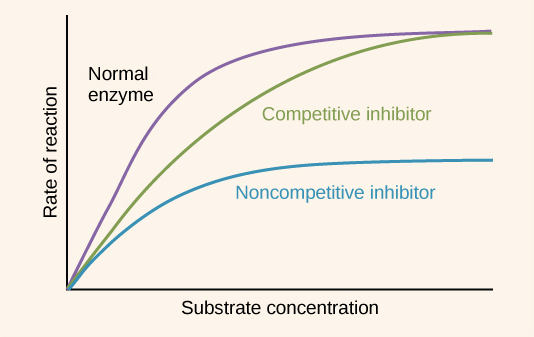
Compare non-competitive inhibitors to competitive inhibitors.
C1.1.14 and C1.1.15
Non-competitive inhibitors do not have a similar shape and chemistry to the substrate, while competitive inhibitors do have a similar shape and chemistry to the substrate. Accordingly, non-competitive inhibitors do not bind to the active site of the enzyme (they bind to the allosteric site), while competitive inhibitors do bind to the active site.
Non-competitive inhibitors do not compete with the substrate directly, but competitive inhibitors do.
Non-competitive inhibitors prevent reactions by changing the structure of the active site, while competitive inhibitors prevent reactions by preventing the substrate from binding with the active site.
The reaction rate with non-competitive inhibitors are not significantly impacted by increasing substrate concentration. It may increase the reaction, but only because it increases the rate of reaction for enzymes not inhibited. The reaction rate with competitive inhibitors are impacted by increasing substrate concentration because it decreases the chance that the competitive inhibitor can bind to the active site.
Outline the mechanism and benefit of feedback inhibition.
C1.1.16 (AHL): Competitive inhibition as a consequence of an inhibitor binding reversibly to an active site.
Feedback inhibition is a process where the last product in the biochemical pathway is a non-competitive inhibitor of the first allosteric enzyme in the pathway. In other words, the final product of the process binds to the first allosteric enzyme, which changes the shape of the enzyme. As a result, the enzyme changes shape, and the reaction cannot occur. Because the first reaction is stopped, all other reactions also stop. The process resumes when the concentration of the final product is low and no longer inhibits the first reaction in the pathway.
Feedback inhibition is reversible, so the reaction can be checked. Additionally, feedback inhibition allows metabolic reactions to speed up or slow down. It prevents an oversupply of the final product by preventing the reaction if there is too much product.
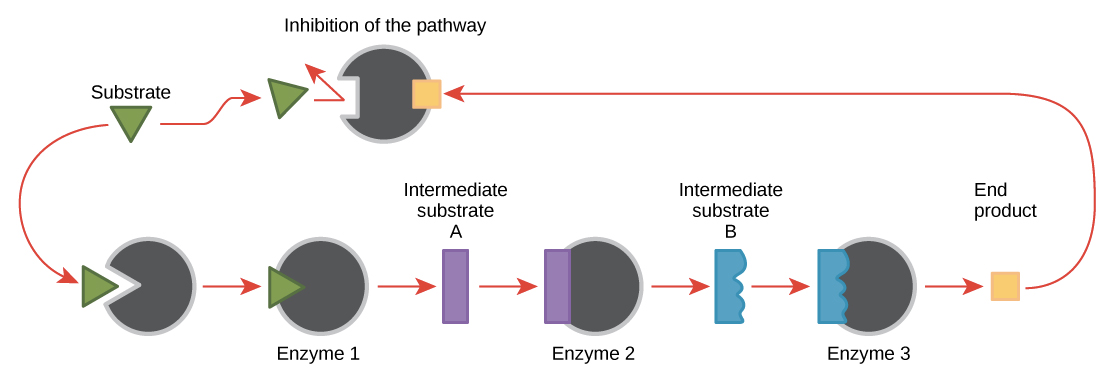
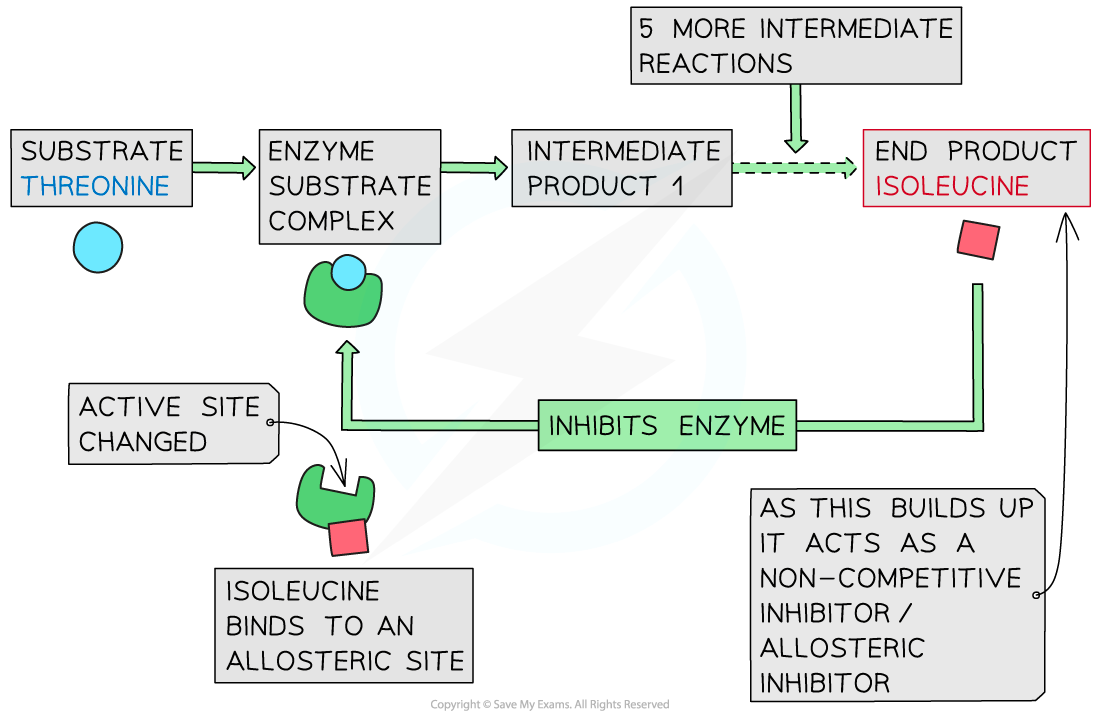
Describe end-product inhibition of the threonine to isoleucine metabolic pathway.
C1.1.16 (AHL): Competitive inhibition as a consequence of an inhibitor binding reversibly to an active site.
Threonine is an essential amino acid that is converted to the aminoacid isoleucine through enzyme-catalyzed reactions.
When there is excess isoleucine, it binds to the allosteric site of threonine deaminase (Isoleucine acts as a non-competitive inhibitor of threonine deaminase), which changes its shape. Thus, threonine cannot catalyze the first reaction in the pathway, preventing all subsequent reactions.
When isoleucine levels drop, it releases threonine, which restarts isoleucine production.
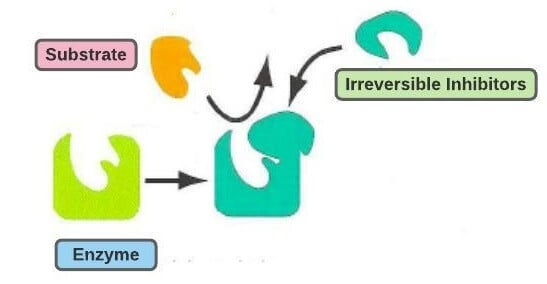
Outline the cause and consequence of mechanism-based inhibition.
C1.1.17 (AHL): Mechanism-based inhibition as a consequence of chemical changes to the active site caused by the irreversible binding of an inhibitor.
In mechanism-based inhibition, competitive inhibitors permanently bond to an enzyme’s active site.
This is an irreversible (permanent) binding, so the substrate can never access the active site.
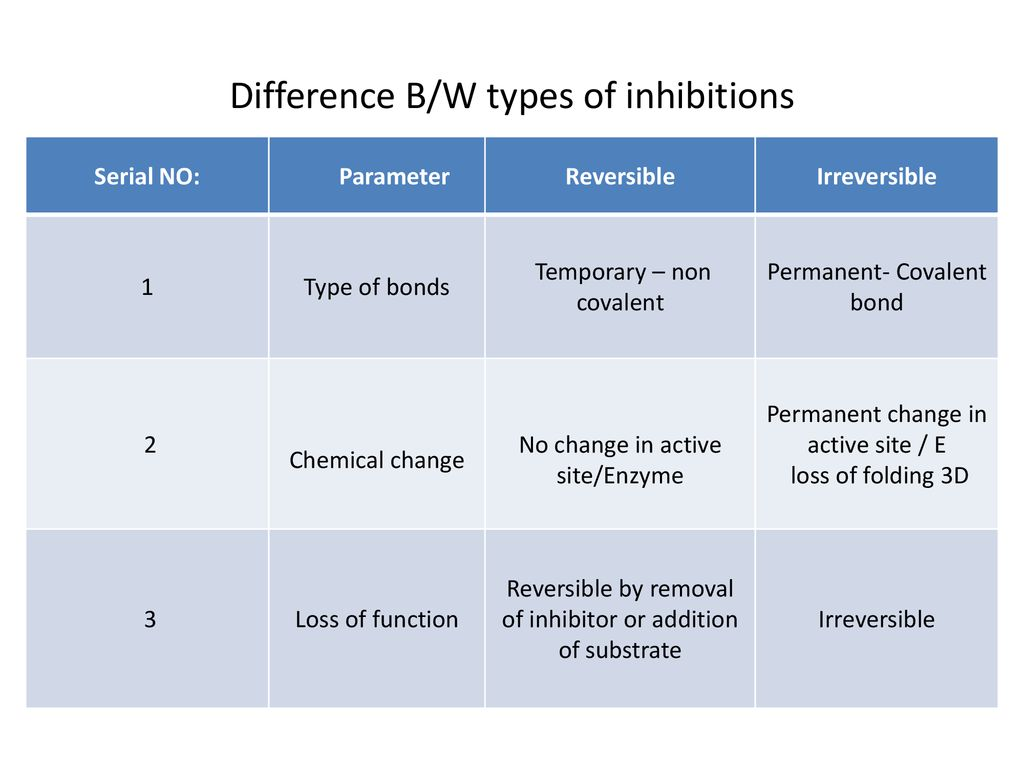
Compare reversible and irreversible enzyme inhibition.
C1.1.17 (AHL): Mechanism-based inhibition as a consequence of chemical changes to the active site caused by the irreversible binding of an inhibitor.
Reversible enzyme inhibition involves non-competitive inhibitors that bond to the allosteric site, while irreversible enzyme inhibition involves competitive inhibitors.
Reversible enzyme inhibition only temporarily inhibits chemical reactions by dissociating from the enzyme, while irreversible enzyme inhibition permanently inhibits chemical reactions.
Reversible inhibition binds through non-covalent bonds, while irreversible inhibition binds through covalent bonds.
Describe mechanism-based inhibition using penicillin as an example.
C1.1.17 (AHL): Mechanism-based inhibition as a consequence of chemical changes to the active site caused by the irreversible binding of an inhibitor.
Penicillin is an example of a mechanism-based inhibitor. Penicillin binds to transpeptidase, an enzyme that helps construct bacterial cell walls. As a result, penicillin prevents bacteria from building cell walls, which causes the bacterial cells to undergo lysis and die.
Penicillin is an inhibitor that performs mechanism-based and irreversible inhibition.