Orgo Lab 2: Nitration
1/14
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
15 Terms
What is a EAS reaction?
electrophilic aromatic substitution
What are the 3 steps of a nitration?
1. Generation of the electrophile
Electrophilic attack on aromatic system
Re-aromatization
What is the solvent of a nitration reaction?
H2SO4
EAS reactions are generally what order reactions?
Second order
Rate law for EAS reaction
K[arene][electrophile]
What is the nitro functional group?
NO2-
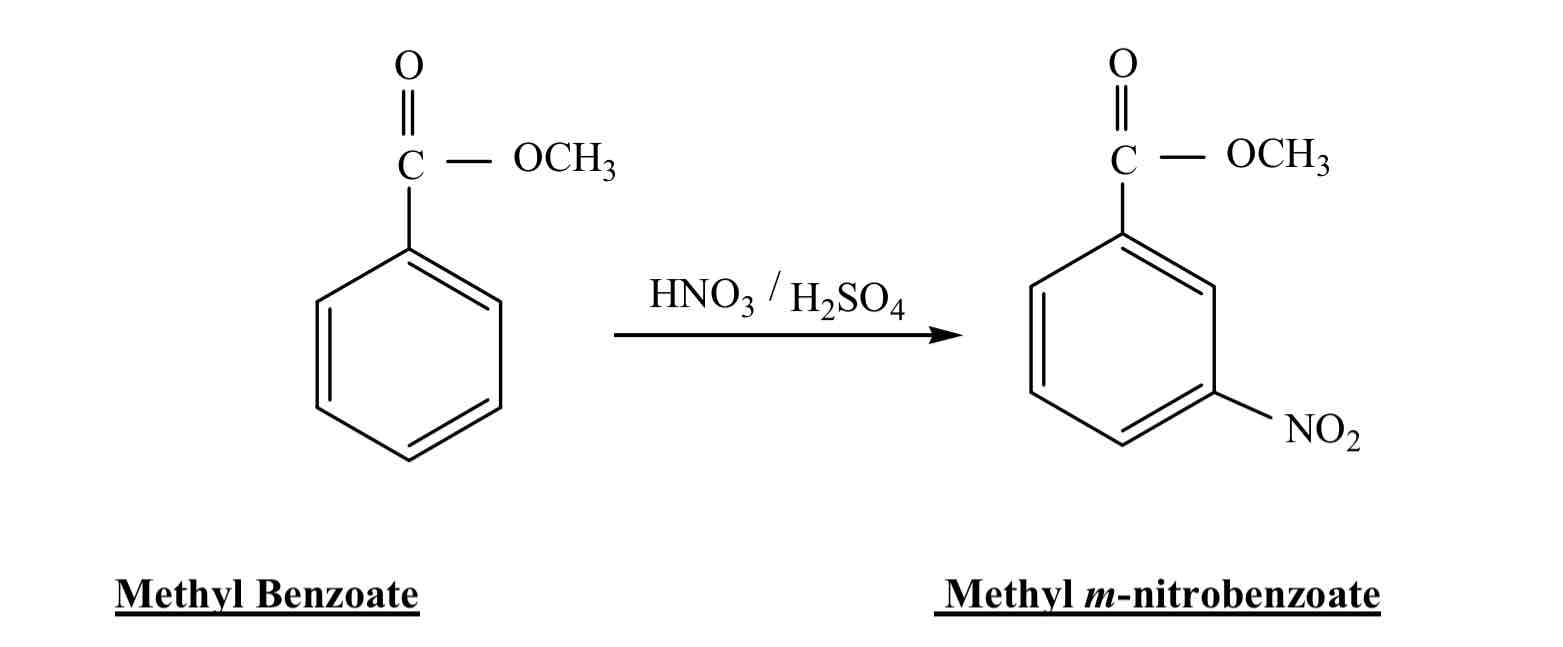
Which has higher mw, mp, bp, methyl benzoate or methyl nitrobenzoate?
Methyl m-nitrobenozoate
The electrophile in the reaction is there nitronium ion which is generated _____ or in the reaction mixture
In situ
Overall reaction for nitration
HO-NO2 + H-HSO4 —> NO2 + HSO4 + H2O
True or False: Higher the temperature, greater will be the amounts of dinitration products formed.
True
An acid spill is neutralized using
Solid sodium carbonate or bicarbonate
Concentrate nitric acid and sulfuric acid are both
Strong oxidizers and high corrosive
What analysis will be used in this lab?
TLC analysis
For the TLC Solent it will be a _ Hexane to _ EA
4:1
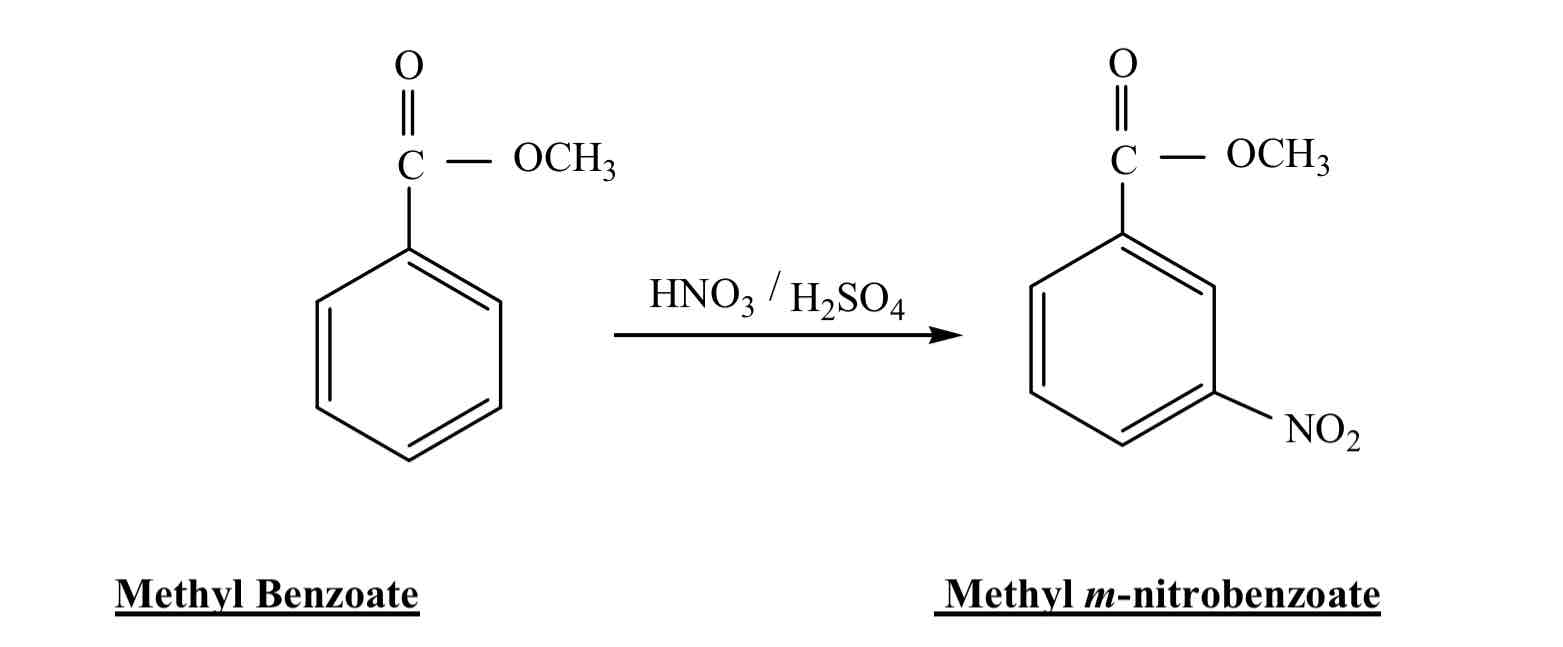
Which will travel further on the TLC plate, Methyl Benzoate or m-nitromethyl benzoate?
Methyl benzoate