SAS Exam 2 - M
1/103
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
104 Terms
common upper airway diseases
Brachycephalic Obstruction airway syndrome (BOAS)
laryngeal paralysis
obstruction:
neoplasia
granuloma
foreign body
trauma:
obstruction
wounds
pneumothorax
pneumonmediastinum
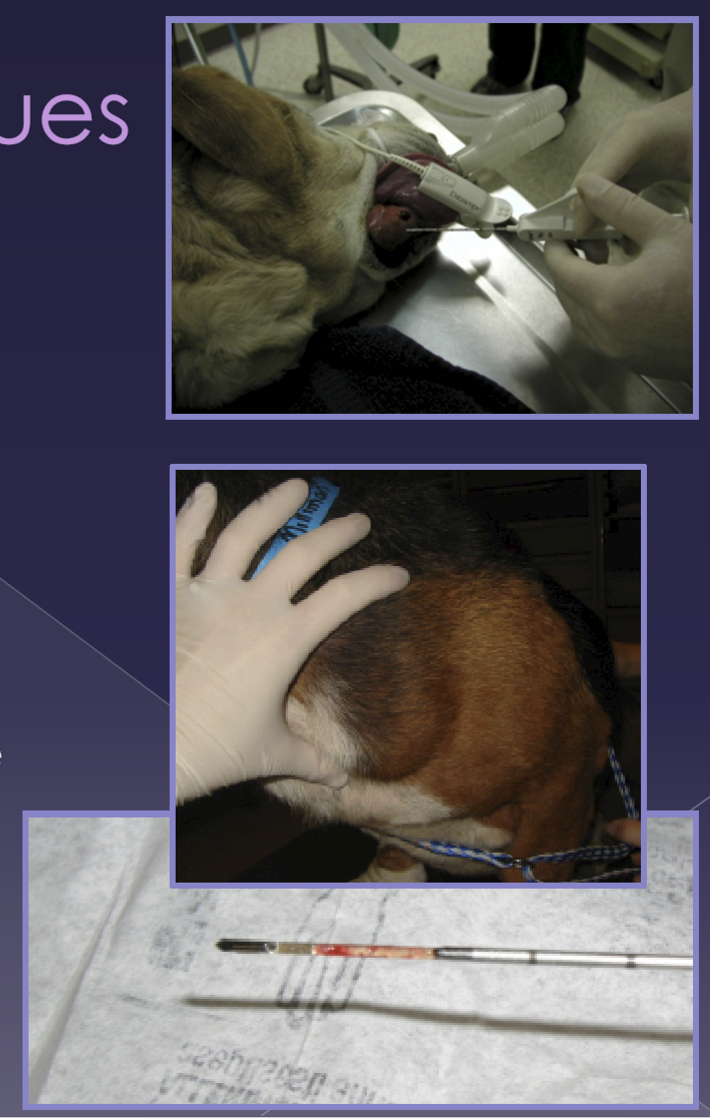
preoperative management for upper airway issues
identify respiratory difficulty
open-mouth breathing
abducted forelimbs - cowboy walk look
labored breathing
restlessness
muddy color cyanotic look
use minimal restraint
oxygen support - important
sedation - to calm them down so they can take better breaths
Butorphanol
Acepromazine
± cooling - especially bulldogs
Anesthetic management for upper airway issues
extreme anesthetic risk!
greatest danger: induction and recovery
pre-oxygenation - always
examine the upper airway at induction
rapid endotracheal intubation (ET tube)
be prepared for a temporary tracheostomy
recovery
calm, quiet
oxygen
ventilation support
dont take tube out until they are chewing the trach tube
Brachycephalic obstruction airway syndrome (BOAS)
these dogs walk around and you can hear them breahening / snoring
signalment
Brachycephalic breeds
compressed face
poorly developed nares
distorted nasopharynx
redundant tissues
often young / early age
syndrome
stenotic nares
soft palate elongation
laryngeal saccule eversion
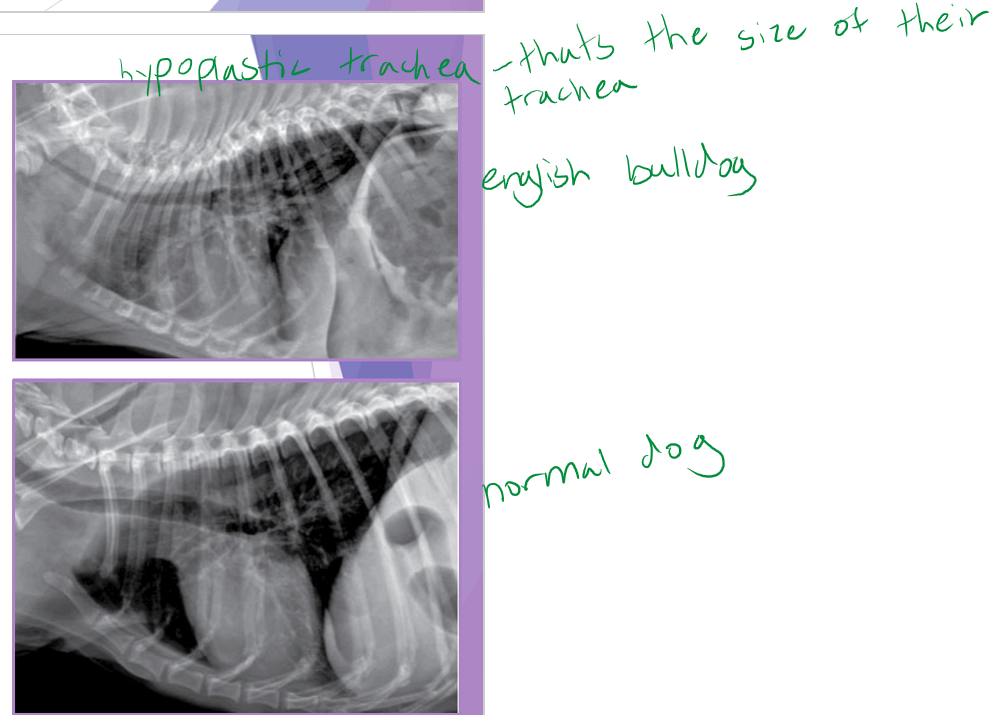
hypoplastic trachea
clinical signs worsen with time - treat early
clinical signs
stertor (increased nasal sounds)
stridor (high pitched wheezing)
coughing, gagging
exercise intolerance
harder to breath at night so restless sleeping pattern
collapse
dyspnea
BOAS diagnostic workup
Blood work - usually boring
neutrophilia with left shift (if aspiration pneumonia)
thoracic radiographs
rule out aspiration pneumonia
identify a hypoplastic trachea
identify concurrent tracheal collapse
± cervical radiographs
identify an elongated soft palate
evaluate for any cervical masses
evaluate for any cervical tracheal collapse
airway examination
one anesthetic episode
perform when ready to do surgery

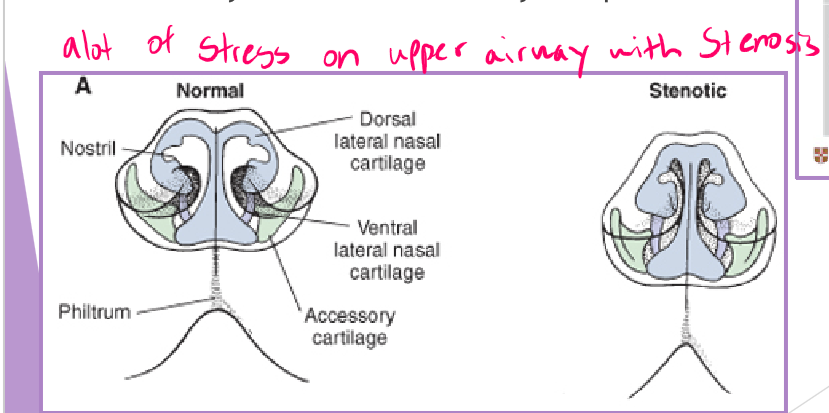
BOAS stenotic nares
abnormally narrow nostrils
congenital malformation of nasal cartilages
why a concern
normal resistance to airflow is 76-80%
airway pressures increase with narrowing
airway tissues will eventually collapse
surgical management - open the nares
multiple techniques
cartilage resection / suture anastomosis
cartilage amputation (traders technique)
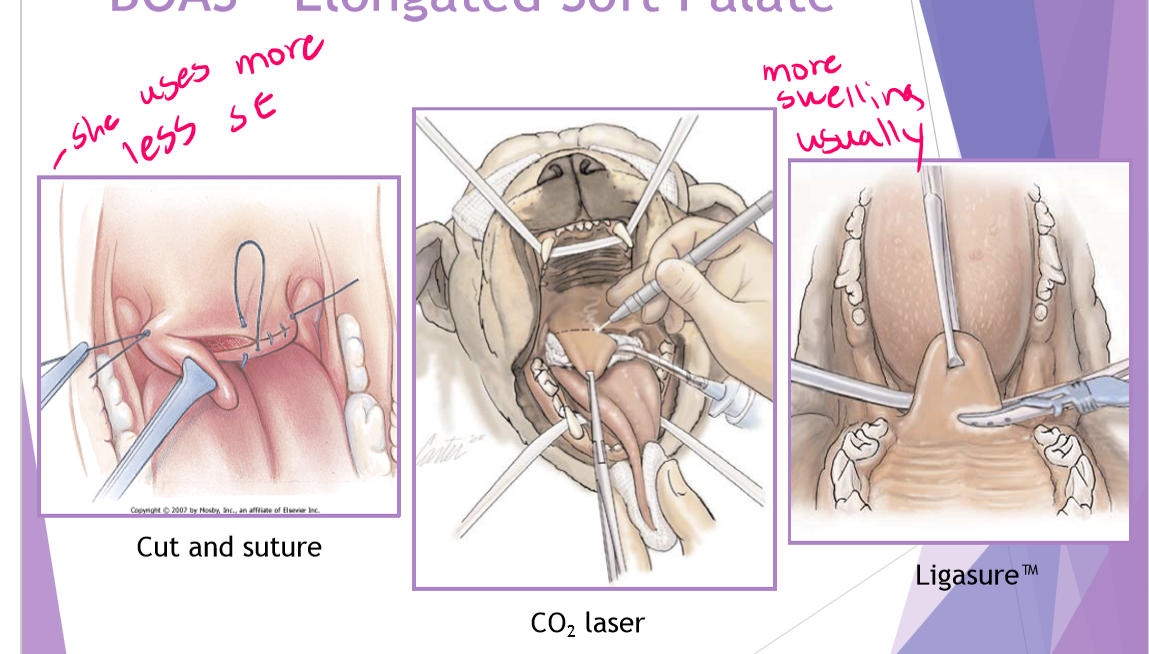
BOAS Elongated soft palate
palate extends >1-3mm beyond the epiglottis
subjective evaluation - caution
can use tonsils as a guideline
laryngeal mucosa becomes inflamed
why a concern
laryngeal edema = airway obstruction
chronic upper airway stress
like having curtains over open window decreased airflow the soft palate drops down over the epiglottis
surgical technique (staphylectomy/palatoplasty) - shortening the soft palate
remove elongated portion of the palate
trim to level of tonsils / just past tip of epiglottis
complications
laryngeal edema
airway obstruction
short term
hemorrhage
aspiration
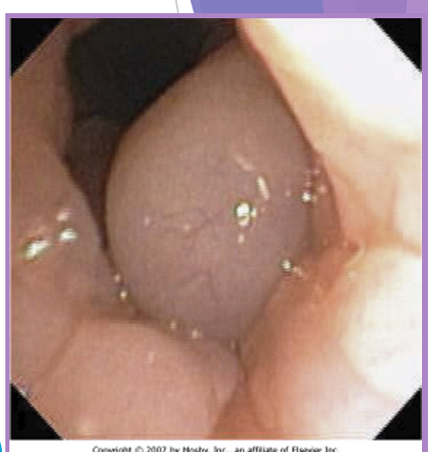
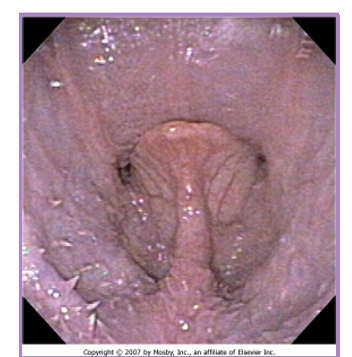
BOAS everted laryngeal saccules
prolapse of mucosa lining the laryngeal crypts
response to chronic high upper airway pressures
least common of the BAS complex
why a concern
further inhibit airflow, increases mucosal irritation
first stage of laryngeal collapse
surgical technique - cut them out but careful of vocal folds
extubate the patient temporarily
grasp and pull with forceps
resect saccule at its base with metzenbaum scissors
bleeding is controlled with direct pressure (from the ET tube)
happens due to chronic stress
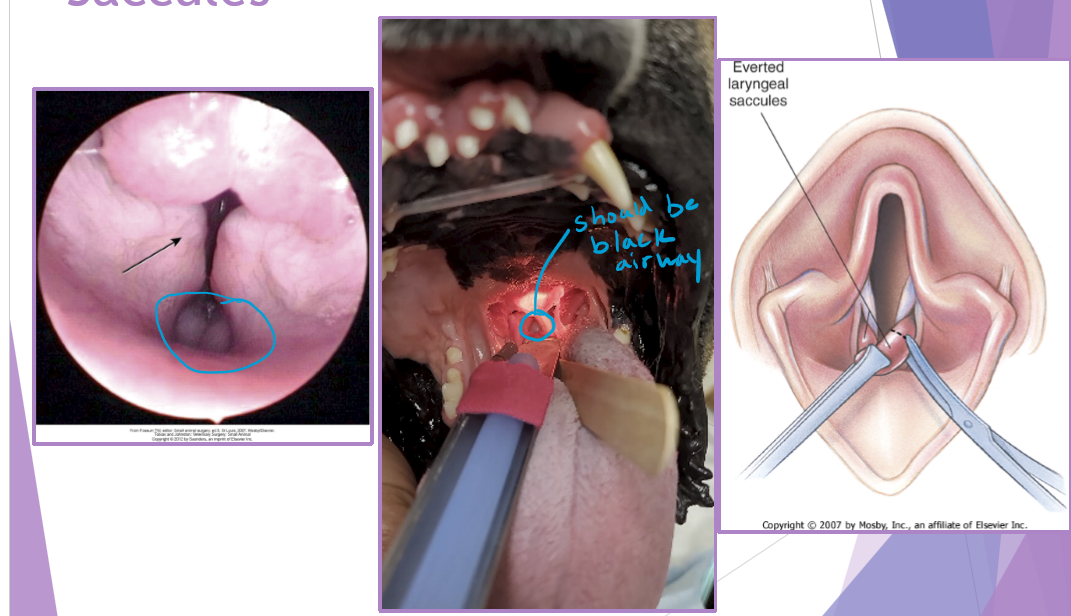
BOAS
Hypoplastic trachea
tracheal diameter to small: thoracic inlet <0.2 - have to do after a year old b/c will grow
cannot surgically correct
laryngeal collapse: secondary due to chronic stress
due to chronic upper airway obstruction / airway resistance
3 stages
I - everted laryngeal saccules
II - I + collapsed cunieform cartilages (red)
III - I + II + collapsed corniculate cartilages (green) - everything collapsed down cant breath at all
treatment
laryngectomy
permanent tracheostomy
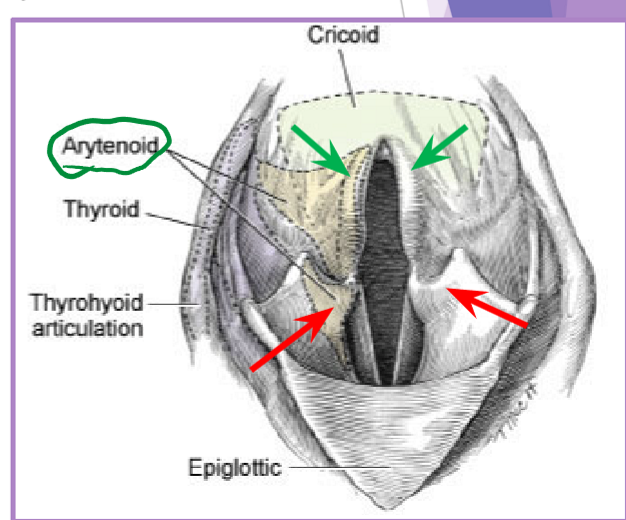
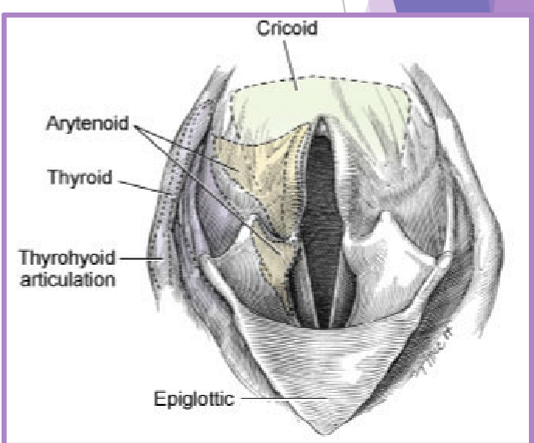
Laryngeal paralysis
complete or partial failure of the arytenoid cartilages
failure of cartilage to open up during inspiration
recurrent laryngeal nerve
cricoarytenoideus dorsalis muscle
congenital
Rottweiler, Dalmation, White-coated German shepherd, Great pyrenees, Leonburger, Bull terrier, Bouvier des flandres
acquired - most of the time
causes
idiopathic****
trauma
systemic disease
Iatrogenic
signalment
large breed dogs > medium / small breed
labrador, irish setter, saint bernard
clinical presentation
inspiratory stridor
voice change
exercise intolerance
coughing, gagging
anxious
collapse
± generalized weakness, muscle atrophy (goolp)
clinical signs worsen with time!
often starts as unilateral then progresses to bilateral paralysis
voice change unilateral
bilateral = more clinical signs
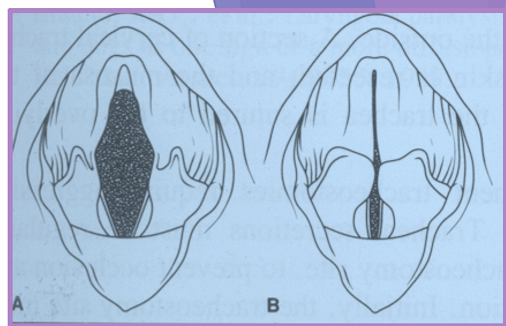
Laryngeal paralysis examination
light plane of anesthesia (induction)
opioid premedication + propofol ± doxapram
evaluate for purposeful movement of arytenoid cartilages
abduction of arytenoids and vocal folds on inspiration
caution: fluttering, paradoxical movement
surgical techniques
complicated anatomy
goal:
reduce the obstruction within the airway
reduce airway resistance
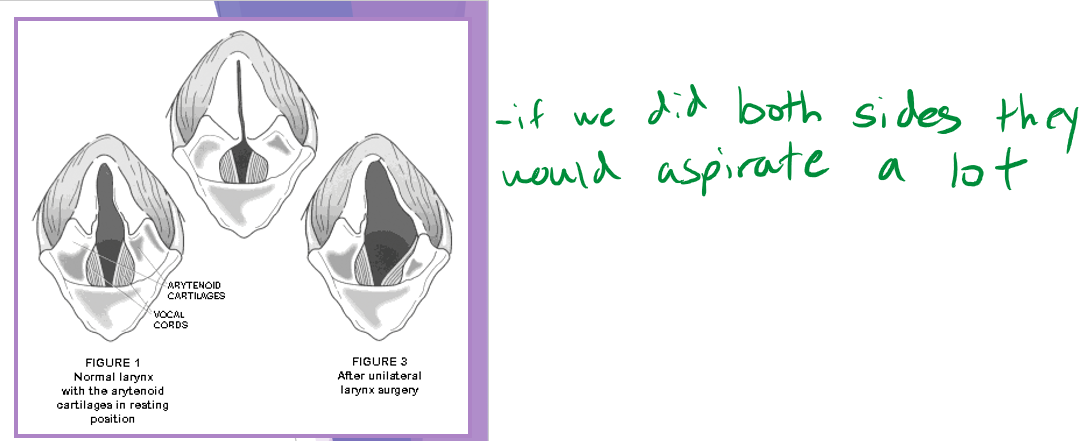
unilateral arytenoid lateralization - most common
abducts one side of the arytenoid cartilages - we only do surgery on one side no matter if both are affected
complication with surgery (10-60%)
failure of the procedure
higher with mineralization of cartilages
cartilage fracture
suture breakage
aspiration pneumonia - biggest risk especially first 24hours but life long problem
less of a risk with unilateral lateralization
prognosis
good - 90% improvement in clinical signs
life long risk
aspiration pneumonia
continued heat / exercise intolerance
progression of polyneuropathy - if GOLPP is present
Lung lobectomy
Normal lung volume
right lung - 58%
left lung - 42%
lobectomy: partial or complete removal of a lung
dogs easily tolerate up to 58% removal
compensation occurs via:
hyperinflation of the remaining lung
enlargement of the alveolar air spaces
thinning of the alveolar capillary tissue barrier
indications
partial lobectomy
focal lesion at peripheral ½ to 2/3 of lung lobe
neoplasia
granuloma
bulla
biopsy
complete lobectomy
large amount of purulent material (abscess)
trauma
lung love torsion
large / multifocal lesion
neoplasia
bulla
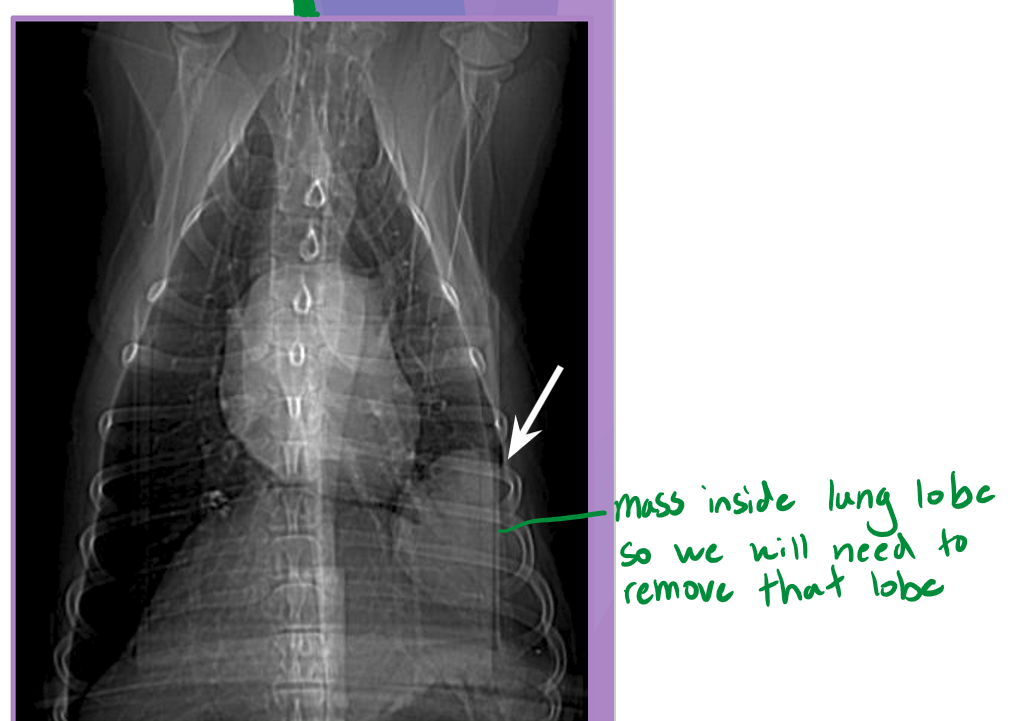
complete lobectomy
intercostal thoracotomy or median sternotomy
remove lung at pedicle
ligate main artery, bronchus, vein
all the way to hiatus
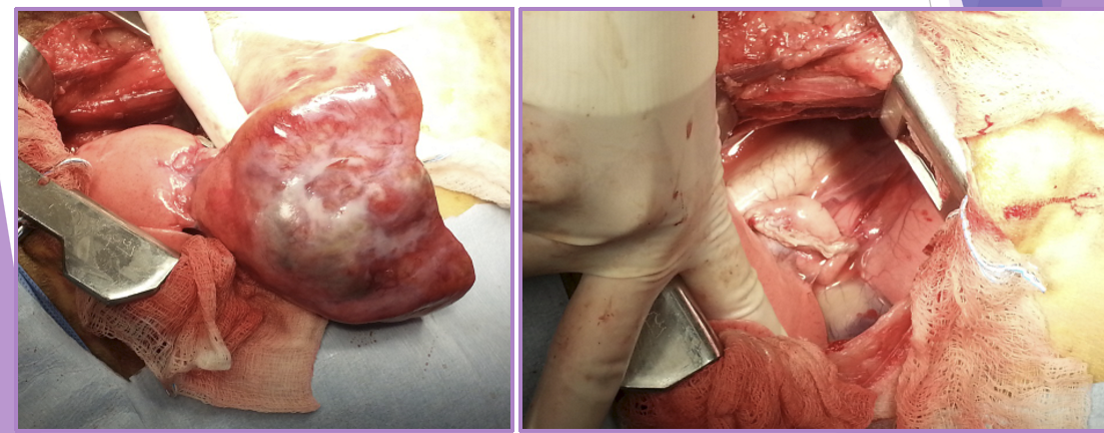
complete lobectomy - Torsion
signalment - deep, narrow chest
right cranial and middle are most common
venous and bronchus obstruction arterial flow remains
lobe becomes congested and consolidated
can be associated with:
chronic respiratory disease
chylothorax
trauma
thoracic surgery
neoplasia
idiopathic*** most common
surgery
complete lobectomy WITHOUT untwisting the lobe
Traumatic and congenital diaphragmatic hernia
very common
continuity of the diaphragm is disrupted
abdominal organs migrate into the thorax
due to an alteration in pressure gradient
acute
shock, associated injuries, respiratory difficulty
chronic
respiratory dyspnea, exercise intolerance, nonspecific (ADR)
signalment: no breed predisposition
tear often occur in weakest area - through muscle
diagnostic evaluation
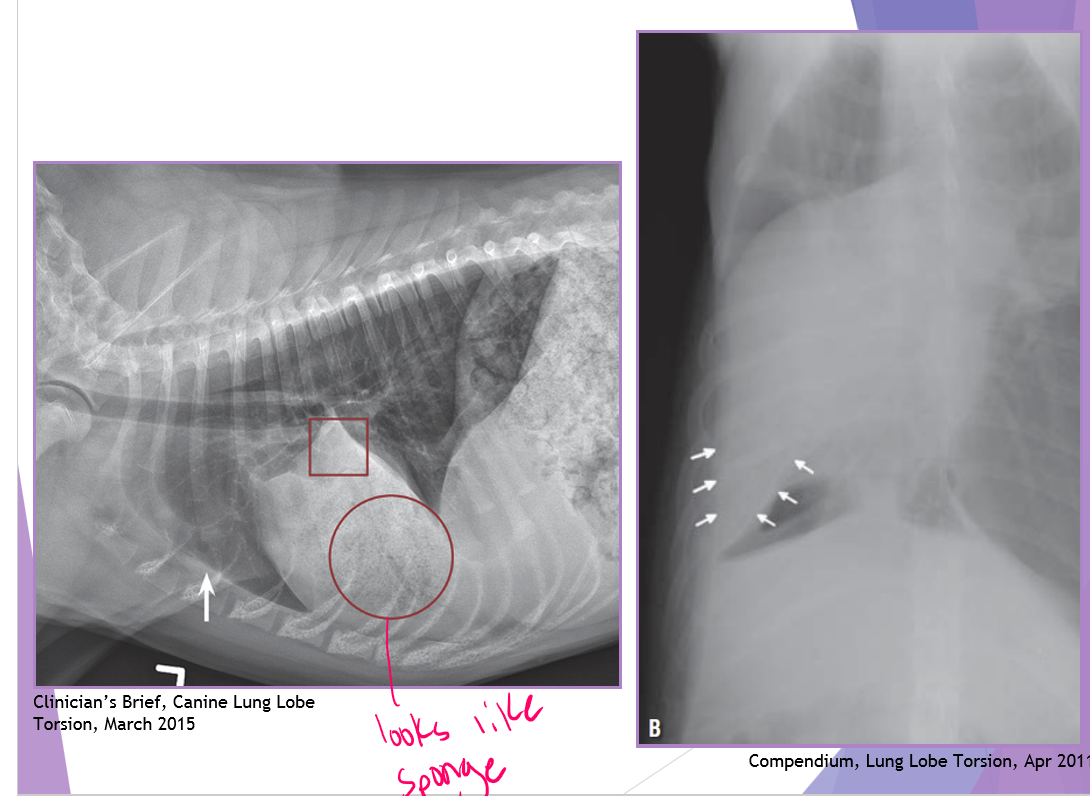
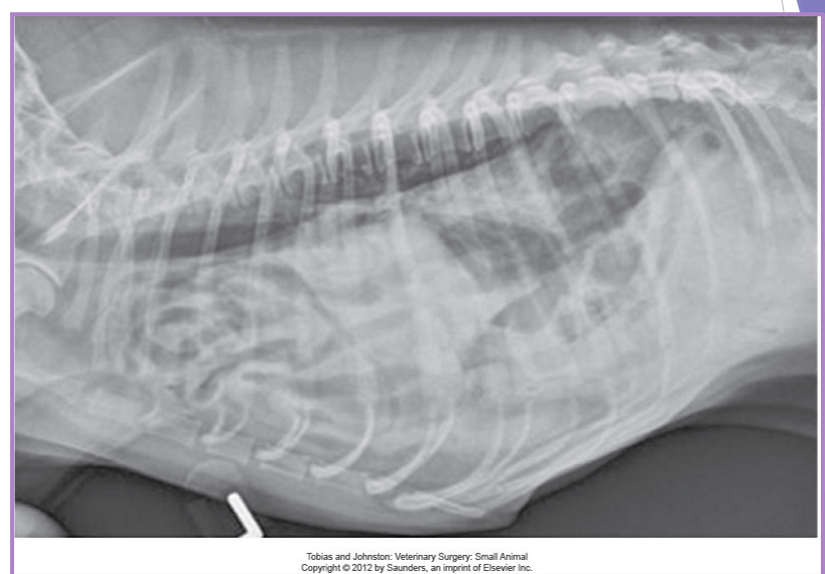
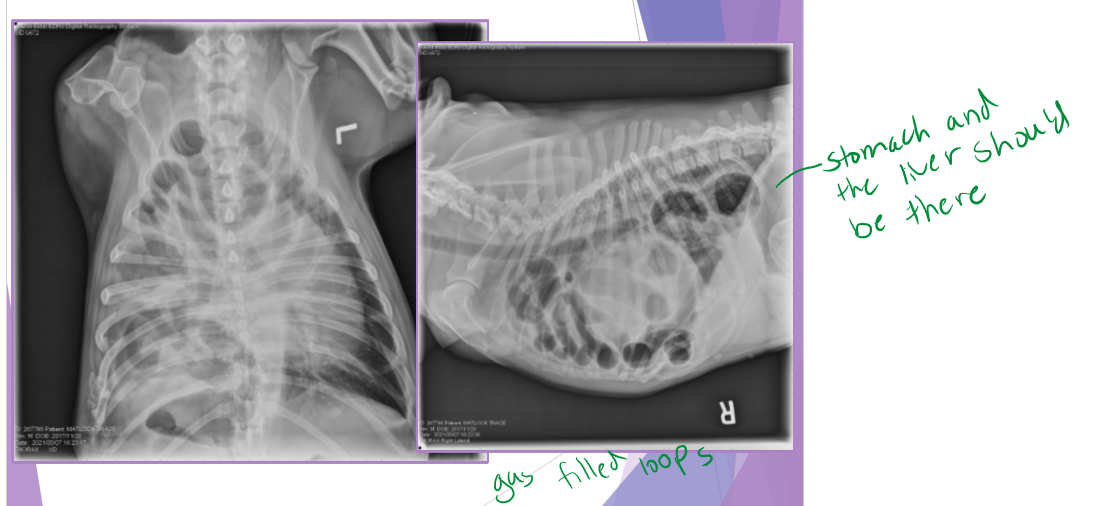
thoracic radiographs (65% accurate)
pleural effusion
gas/soft tissue opacity within the thoracic cavity
stomach against the diaphragm
loss of diaphragm silhouette
ultrasound
CT scan
liver is the most herniated organ, rarely stomach
Diaphragmatic hernia - Traumatic
Surgical repair - be prepared for anything!
abdominal exploratory
identify hernia and carefully reduce contents
caution - adhesions could be present
close diaphragmatic defect
account for tension
freshen edges if chronic
suture defect closed - simple continuous with absorbable suture
3-0 PDS
suture from dorsal (deep) to ventral (superficial)
remove air from thoracic cavity - with needle and syringe and pull air out so lungs can re-expand
do NOT re-expand lungs manually!!!
can cause re-expansion pulmonary edema
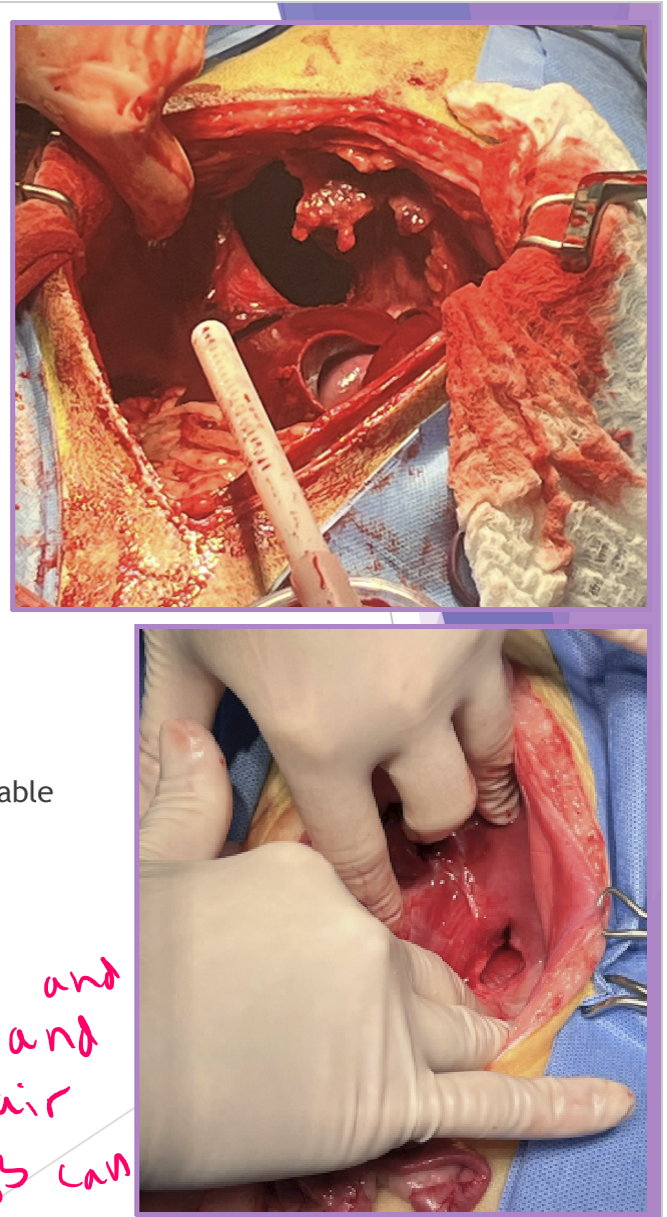
Diaphragmatic hernia - Congenital
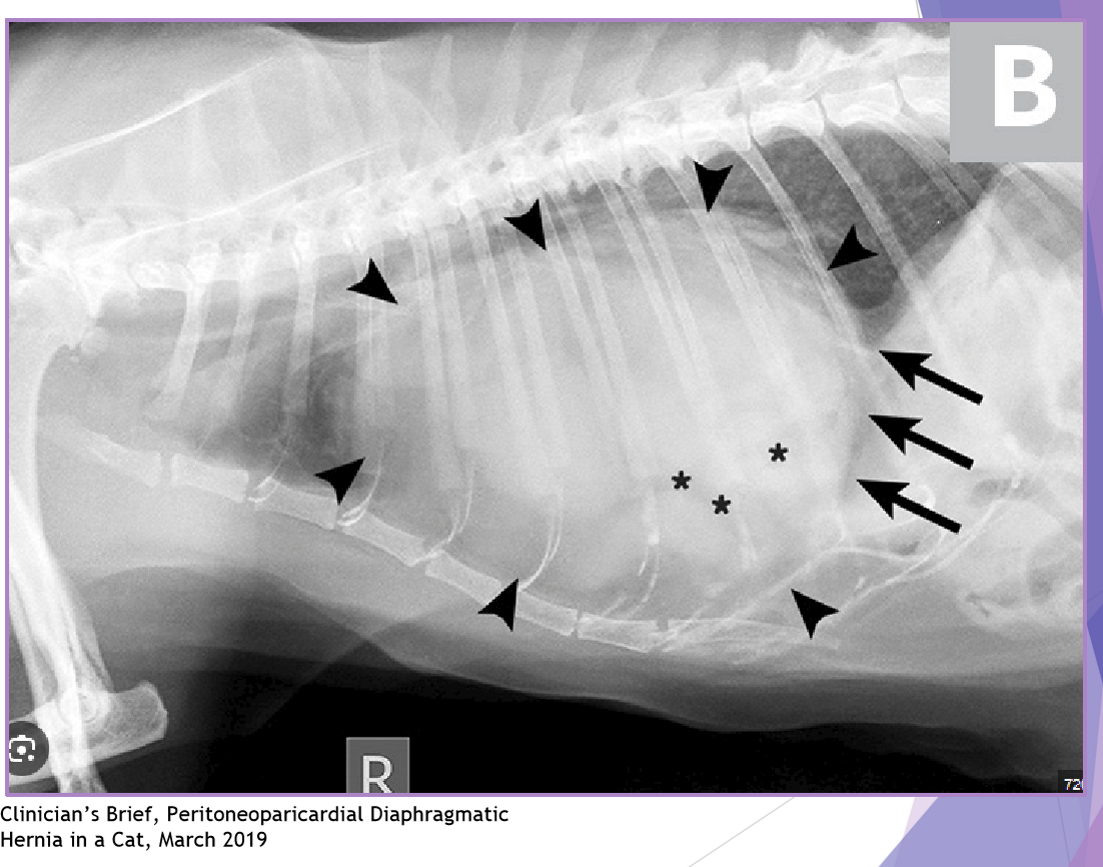
Peritoneopericardia d. hernia (PPDH)
congenital common cavity between pericardium and peritoneal cavity
breeds: cocker spaniel, weimaraner, himalayan, DLH
clinical signs:
asymptomatic - most common
respiratory dyspnea
look for other congenital defects
diagnostic evaluation: thoracic radiographs
enlarged, globoid cardiac silhouette
± gas opacity in the cardiac silhouette
pericardial effusion
surgical repair
perform as early as possible (8-16 weeks of age)
abdominal exploratory
gently replace abdominal organs
enlarge diaphragmatic defect if needed
do not close the pericardial sac
close the diaphragmatic defect
remove air from thoracic cavity
complications (traumatic and congenital)
re-expansion pulmonary edema
abdominal compartment syndrome
acute respiratory distress
Lymph nodes palpable when enlarged
maxillary
accessory axillary
cervical
femoral
retropharyngeal
sublumbar***
mesenteric***
Lymphadenomegaly
causes
infection
inflammation
neoplasia (metastatic, primary)
systemic disease
localized or generalized
size does not correlate with disease
palpation is important
painful
suppurative lymphadenitis (infection)
non-painful
lymphoid neoplasia
fixed
metastatic neoplasia
fungal
fine needle aspiration lymph nodes
screening tool
performed as a first step
cellular sample only
specific but not sensitive
etiologies
bacterial
fungal
neoplasia - mesenchymal, epithelial
why biopsy lymph nodes
Diagnosis
non-diagnostic FNA
neoplasia
culture
disease staging
develop treatment plans
Needle (tru cut) biopsy techniques
septic technique
large bore (14-16g)
indication
larger node
safe location
core of tissue
fairly small sample size
easy / quick
expensive
Incisional (wedge) biopsy techniques
indications
regional anatomy concern
smaller size of node
more difficult location
aseptic technique
stabilize node
wedge-shaped incision
capsule sutured closed
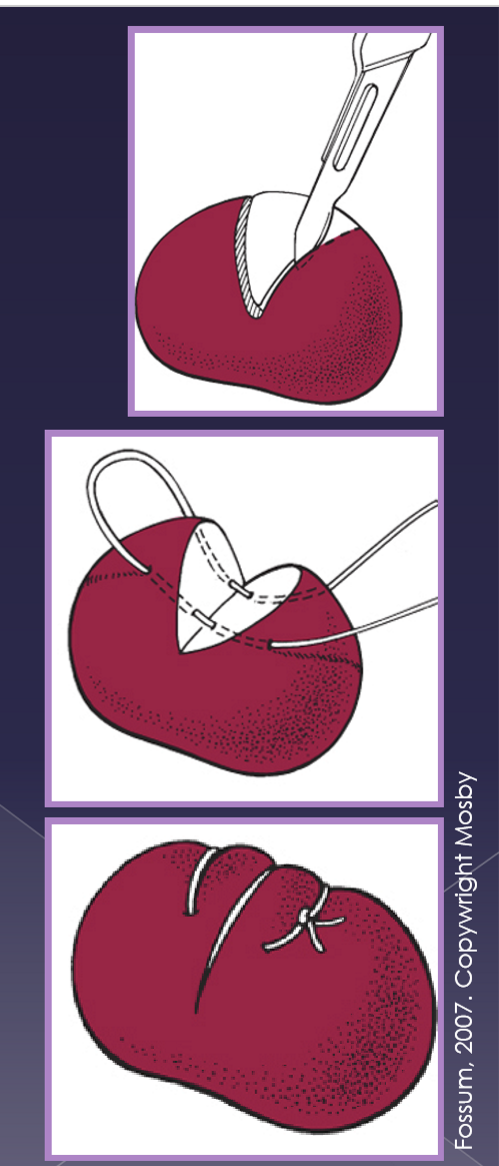
Excisional (lymphadenectomy) biopsy technique
indications
smaller node
evaluate for metastasis
does not prevent metastasis
aseptic technique
surgical approach to node
dissection of the node
ligation of the blood vessels
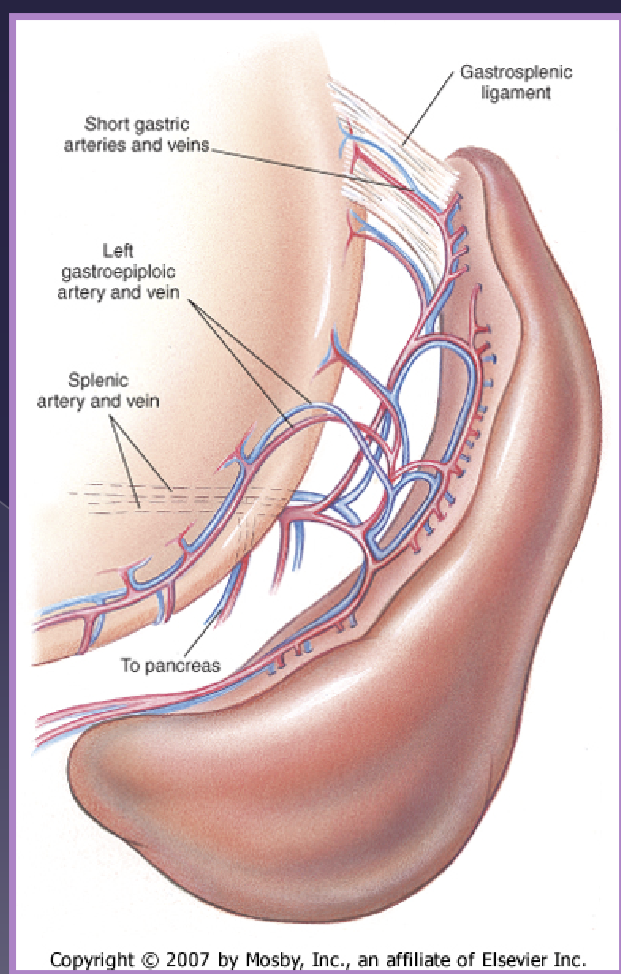
splenic anatomy
gastrosplenic ligament
vascular supply
celiac artery →
splenic artery
A. branch to the pancreas
left gastroepiploic a
short gastric aa
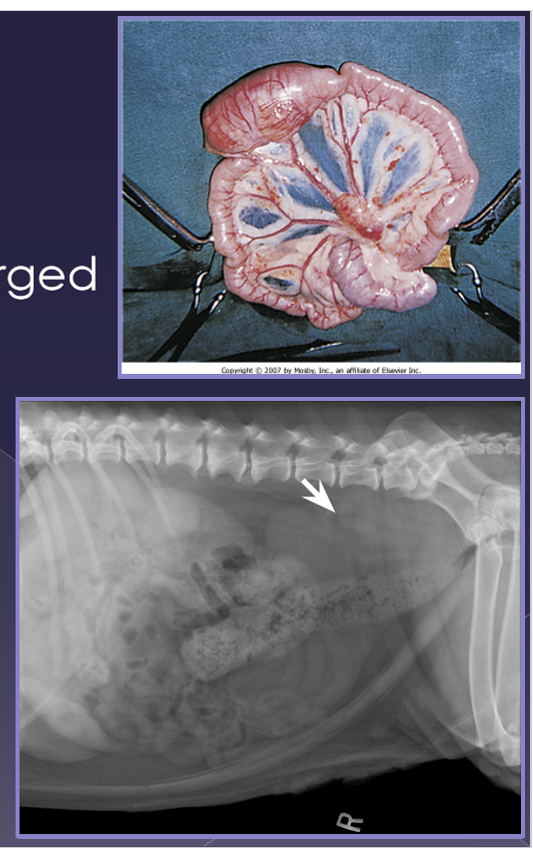
splenomegaly
Diffuse
congestion
splenic torsion*
right-sided heart failure
gastric dilatation - volvulus (GDV)*
drugs
infection*
immune-mediated*
neoplasia-lymphoma
focal
nodular regeneration
hematoma*
trauma*
neoplasia*
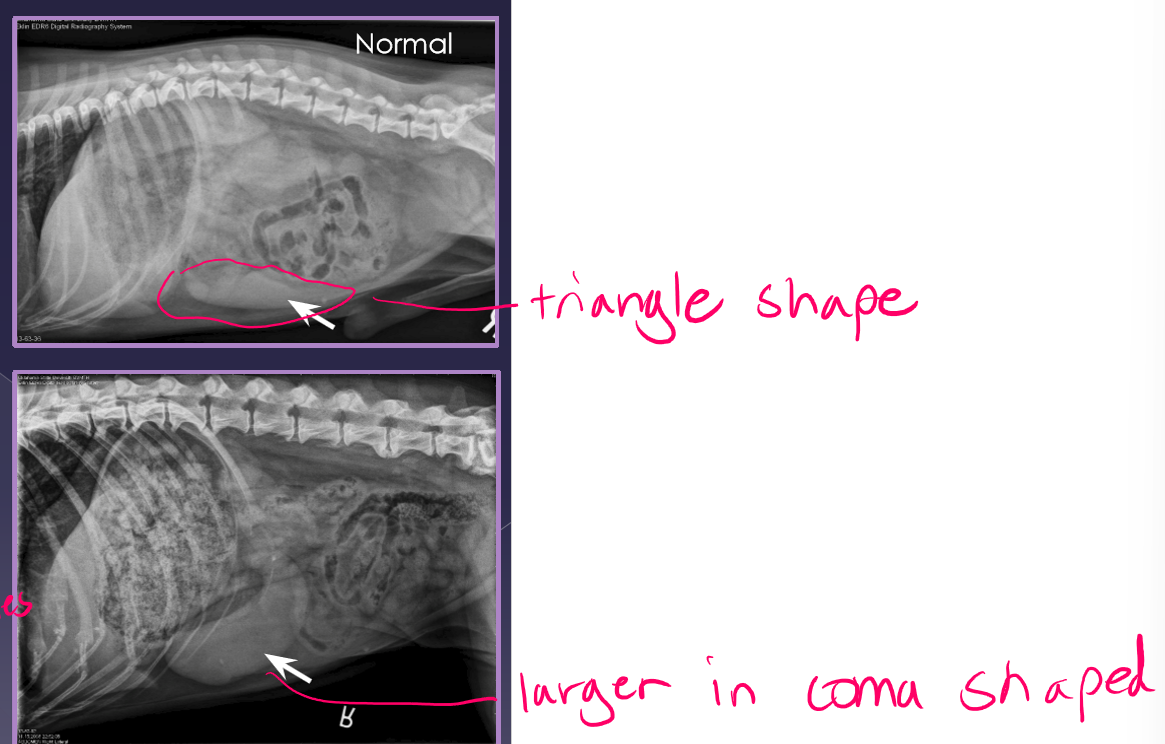
splenic torsion
spleen twists on its vascular pedicle
large breed dogs
uncommon
acute
shock, anorexia, vomiting, diarrhea, abdominal pain, enlarged spleen
chronic
anorexia, vomiting, diarrhea, abdominal pain, enlarged spleen, hemoglobinuria
radiographs
abnormal location
mass effect
gas bubbles
comma-shaped
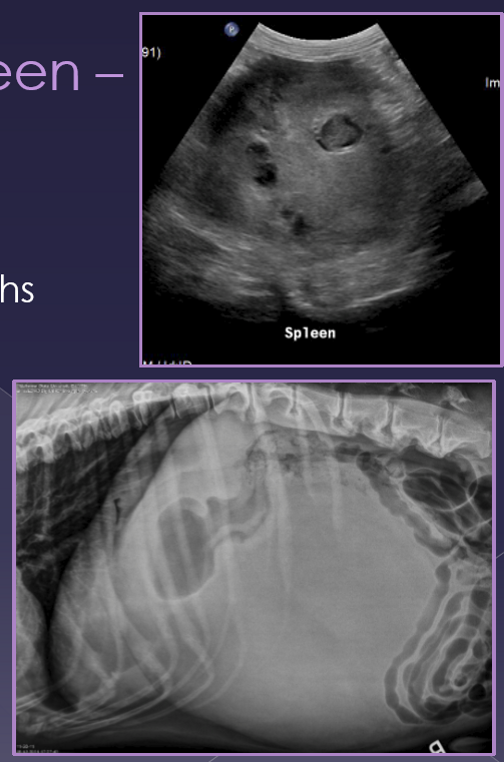
ultrasound
variable echotexture
dilated vessels - gas bubbles
thrombi
treatment
acute more urgent than chronic
cardiovascular stabilization
antibiotics - Unasyn
electrocardiogram - can cause issues with rhyme of heart so want ECG
DO NOT UN-TWIST
Necrotic debris can enter systemic circulation
splenectomy
cannot and should not un-twist
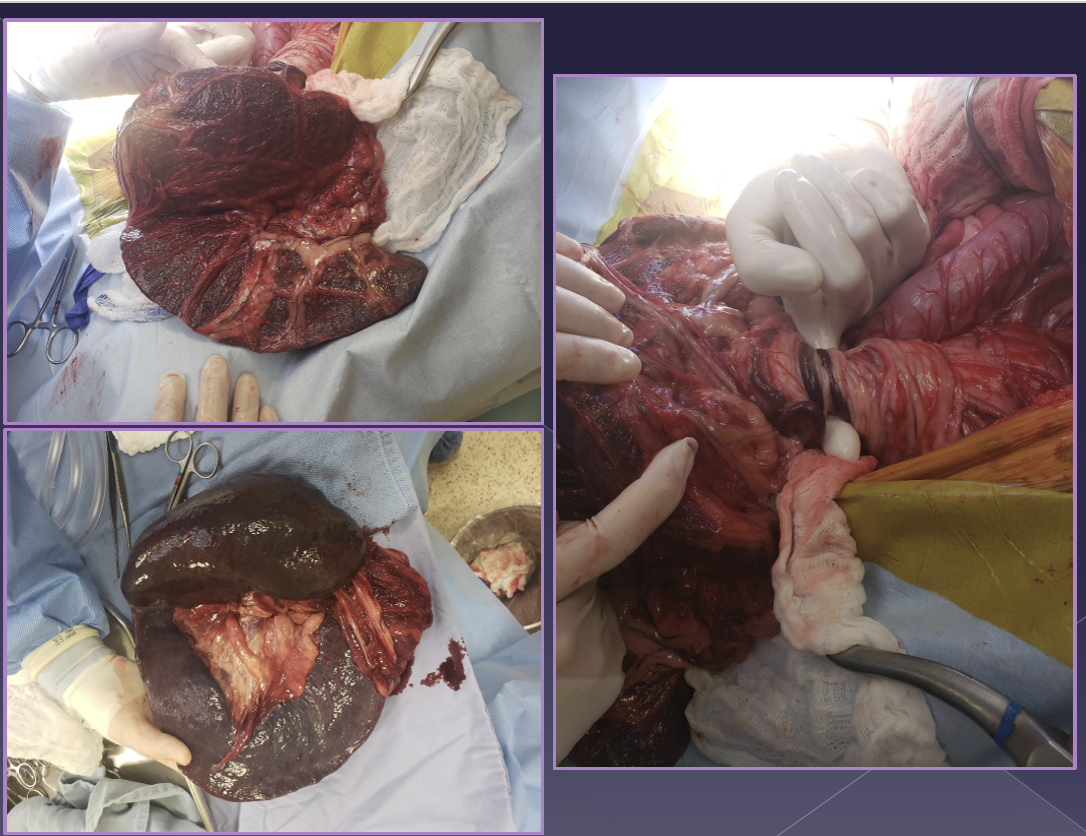
Neoplasia of the spleen
benign or malignant
very common
large breed dogs » small breed dogs
acute
shock, enlarged abdomen, abdominal mass, fluid wave, lethargic, vomiting, abdominal pain
chronic
same as acute but episodic presentation
diagnosis
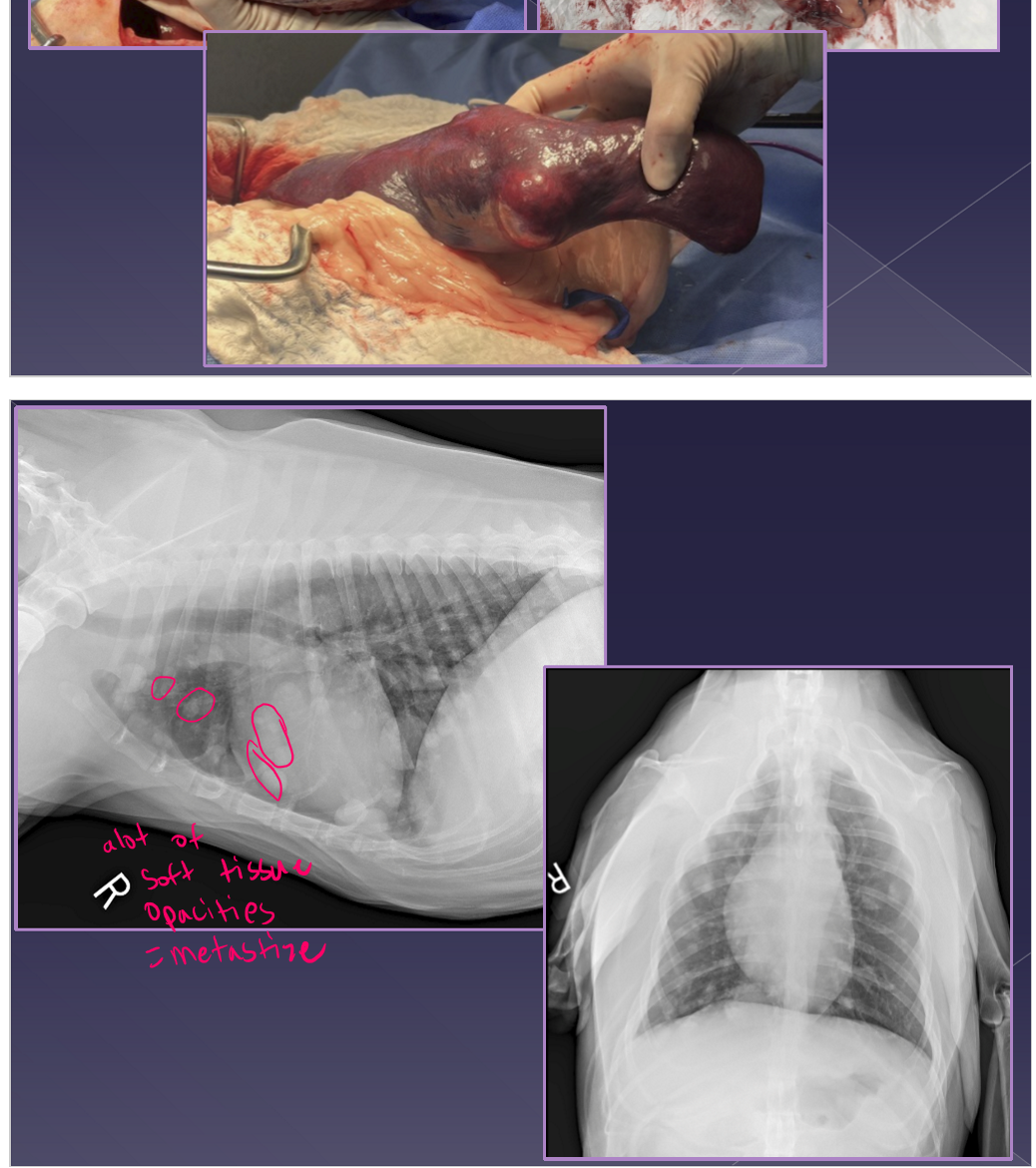
abdominal radiographs
mass-effect
peritoneal effusion
thoracic radiographs
metastasis
ultrasound
mixed echotexture
cavitated lesions
enlarged spleen
spleen neoplasia
differentials
non-neoplastic
hematoma
benign neoplasia
Lipoma / myelolipoma
hemangioma
fibroma
malignant neoplasia
Hemangiosarcoma* - most common
fibrosarcoma
liposarcoma
mast cell tumor
treatment
cardiovascular stabilization
electrocardiogram
± blood transfusion
splenectomy

splenic fine needle aspiration
advantage:
samples obtained percutaneously
cheap, easy
indications:
concern for diffuse disease or definitive mass
caution:
avoid cavitary lesions
major hemorrhage can occur
usually non-diagnostic for neoplastic masses
splenic biopsy
surgical approach to the spleen
obtain more tissue for histopathology
diffuse disease
small/focal mass
obtain tissue for culture
technique
incision into the spleen to remove tissue
close capsule with suture
direct pressure for hemostasis
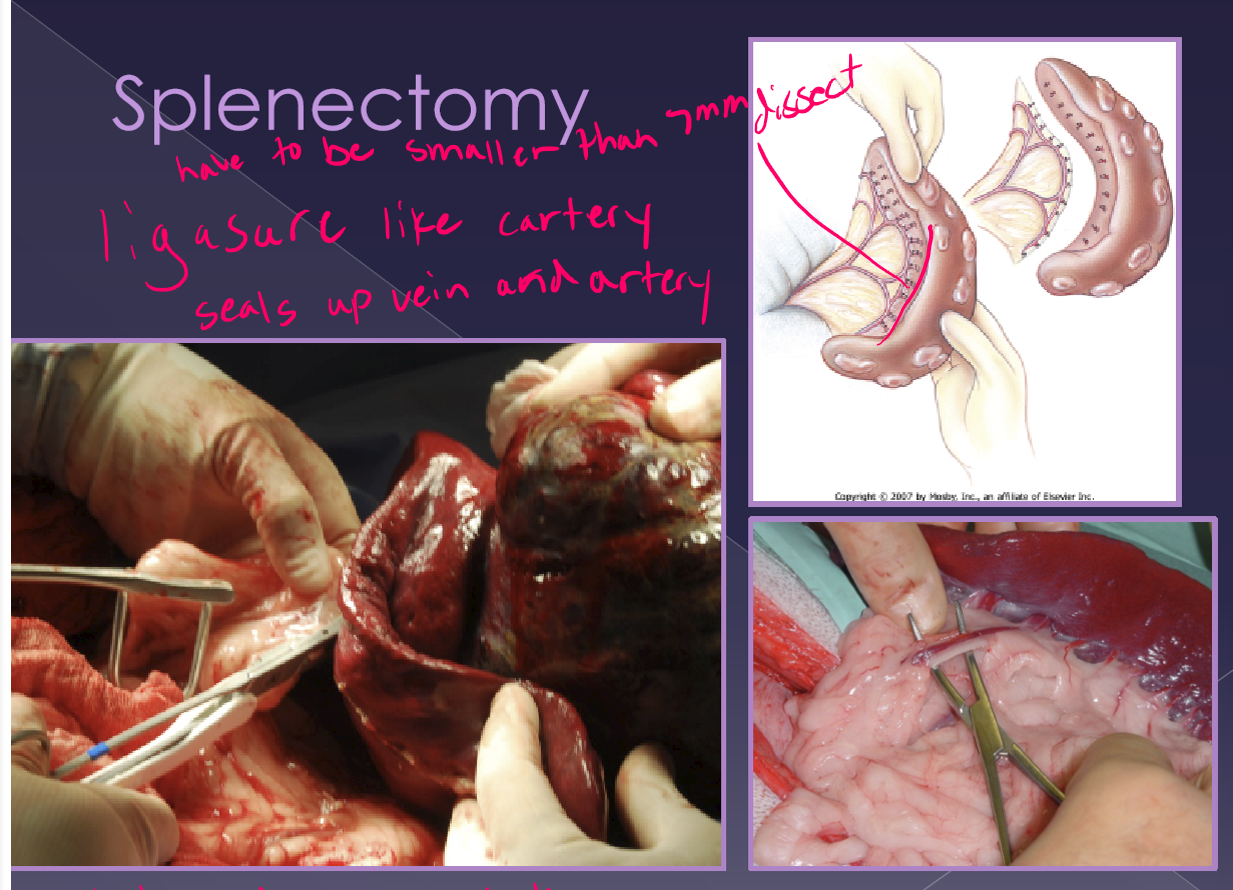
splenectomy
preferred method
indications
neoplasia
severe trauma
immune-mediated disease
concern
large-volume blood loss
classic approach
exteriorize and isolate spleen
dissect, ligate, and divide all hilar vessels
do not damage the short gastric aa.
time consuming
more option for hemorrhage
alternate approach
abdominal exploration
exteriorize and isolate the spleen
identify the splenic artery and vein distal to:
pancreatic branch
short gastric aa
left gastroepiploic a
quicker
ok for normal spleen with no adhesions
splenectomy complications and post op considerations
hemorrhage
hemoabdomen prior to surgery
breakdown of adhesions at surgery
resist this temptation
mass rupture with handling at surgery
ligature slippage
blood contained within the spleen itself
blood transfusion
electrocardiogram
you will see ventricular arrhythmias
crystalloid/colloid fluid support
oxygen therapy
monitor coagulation profiles (PT/PTT)
oncology patient assessment
history and physical exam
visual inspection
mass palpation
evaluate
gross appearance
consistency
size
mobility
palpation of regional lymph nodes
secondary effects of a tumor present
anemia
hypercalcemia
vomiting / diarrhea
oncology fine needle aspiration
cytological evaluation
cellularity
definitive diagnosis
lymphoma
melanoma
mast cell tumor
supportive information
inflammation often accompanies tumors
oncology biopsy
obtain a diagnosis
need to know tumor behavior
degree of local invasion
metastatic potential
biologic activity (ie. histamine release)
pre or postoperative
will the information affect case management
can i harm the patient
what technique consider
invasiveness of the procedure
potential for intra-cavitary hemorrhage
potential to seed tumor cells
biopsy oncology
Incisional / TruCut
removal of part of the tumor
specific behavior of tumor may affect treatment plan/owners decision
disadvantage
requires a second surgery
seed tumor cells if not careful
Excisional:
remove entire tumor with normal tissue
allows for a single procedure
disadvantage:
surgical excision may not be complete
may remove too move tissue
biopsy incisional vs excisional
Incisional:
large skin mass
fixed mass
mass near important structures
musculoskeletal
Excisional:
small, movable skin masses
internal organs
finances
tumor staging
Diagnostic process
to evaluate for progression / extent of disease
tests are dictated by tumor type
often performed after a diagnosis is made
bloodwork
complete blood count
serum biochemistry panel
urinalysis
radiography
three view thoracic
ultrasonography - abdomen
identify masses / infiltration
obtain samples for cytology
surgical planning
lymph node aspiration
enlarged nodes
draining / sentinel lymph nodes
computed tomography
magnetic resonance imaging
oncology surgical principles
one and done
normal anatomy
less chance of tumor metastasis
easier closure
less chance of surgical spread of tumor
excise all neoplastic tissue
tumor itself
biopsy or cytology tracts
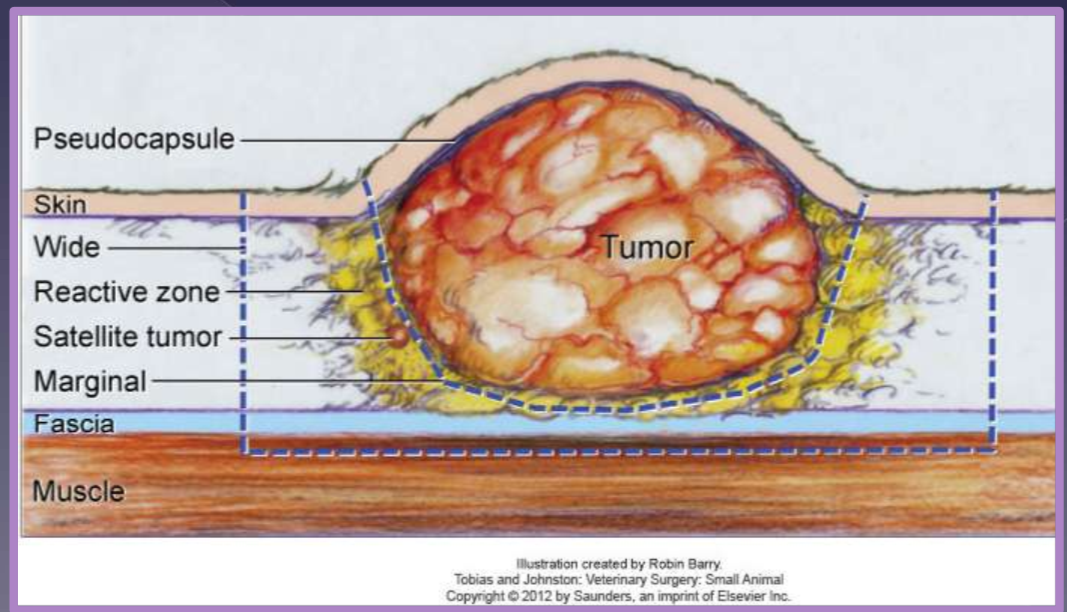
oncology surgical principles margin of excision
Aggressiveness (surgical dose)
intralesional (debulking)
marginal
wide
radical
dependent on
tumor type
tumor grade
location of tumor
intralesional
leave “gross” tumor behind
marginal
just peripheral to pseudocapsule
reactive zone
used for benign
lipoma
satellite tumor
common without a prior biopsy
wide and radical
curative-intent
recommended for solid tumors
excise a margin of normal tissue
deep
1 or 2 facial planes
radical
entire tissue compartment
splenectomy
amputation
mammary chain
oncology post-removal biopsy
Do this! do this! do this!
allows for margin assessment
3D margins: lateral and deep
dictates adjunctive treatment plan
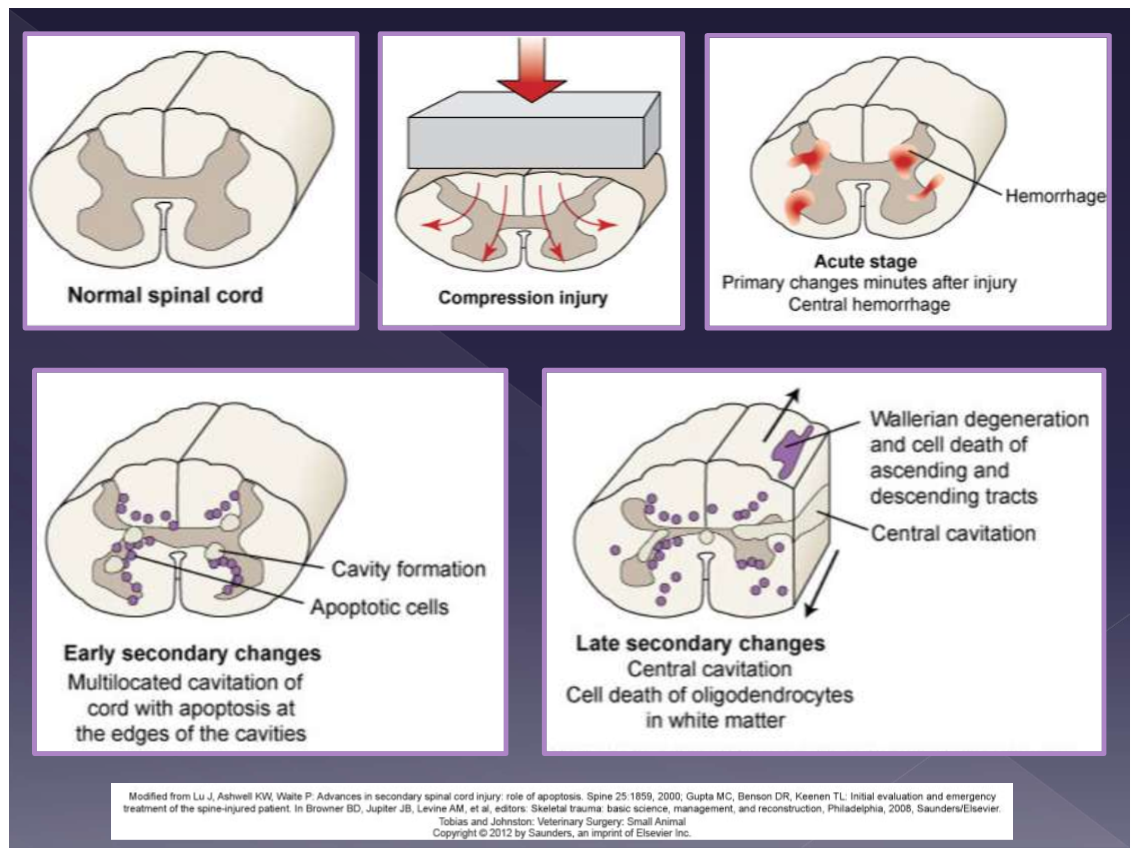
mechanisms of trauma in the CNS
contusion
spinal instability
fracture
luxation
disc herniation
blunt trauma
compression
disc herniation
disc protrusion
spinal instability
fracture
luxation
blunt trauma
localized hemorrhage
spinal cord > brain
primary damage
mechanical trauma
axonal injury
hemorrhage
edema
secondary biochemical effects
demyelination
neuronal and glial cell necrosis
inflammatory response - IL, TNF, NO
severity depends on:
etiology
speed of onset
duration
location
amount of compression
clinical history for CNS issues
environment
how did the trauma occur
duration since trauma
how did the patient appear immediately after the trauma
has the patient had a change in mentation
any medications administered
physical examination for CNS patients
evaluate all body systems
neurologic examination
complete
partial - do not manipulate spinal trauma
gentle palpation of vertebral column
spinal reflexes
severity of neurologic deficits
neurologic localization
brain
spinal cord
goal
determine most likely cause
determine potential prognosis
triage for CNS trauma
treat shock
fluids, ECG, oxygen, maintain patent airway
monitor vitals
decide if spinal trauma is present
defect on paraspinal palpation
neurologic signs in limbs/reflexes
decide if head trauma is present
anisocoria
pupil size
nystagmus
mentation/level of consciousness
bleeding - nose, ear, eye
cushing response
nervous system response to increased ICP
severe cases of head injury/brain herniation
what do you see
increased blood pressure, irregular breathing, reflex bradycardia
Treatment - head trauma
serial neurologic exams
keep head elevated
fluid therapy
oxygen
ventilatory support, if needed
monitor electrocardiogram
pain medications - opioids
mannitol (0.5-1 g/kg) over 20 minutes - removes edema in brain
osmotic diuretic
can do 2-3 doses
Dexamethasone (0.1 mg/kg once)
follow up only after 3rd dose of mannitol, if not effective
decreases edema
surgical intervention
subdural hematoma
depressed skull fracture
debride contaminated / necrotic tissue
stabilize intracranial pressure
procedure
craniectomy
decrease pressure by 15%
durotomy
decrease pressure by 65%
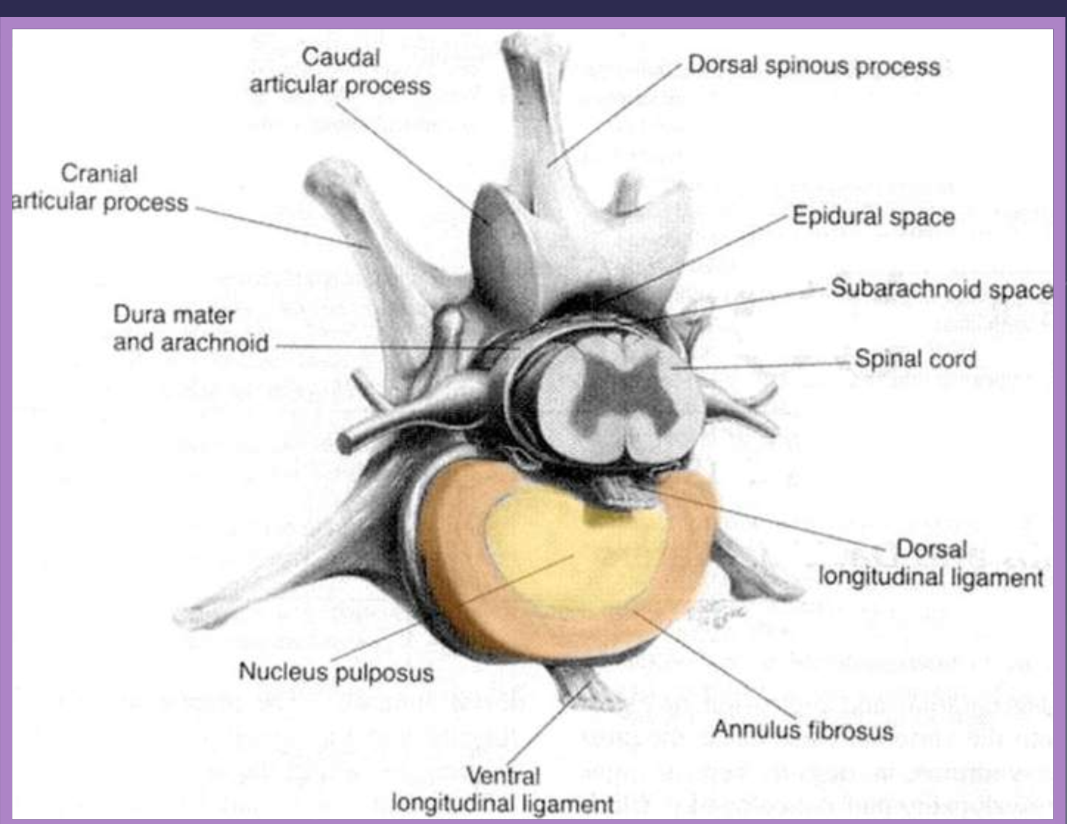
intervertebral disk disease anatomy
#1 most common in general practice
2 components
annulus fibrosus
parallel arrangement of lamellae
thicker ventrally
nucleus pulposus
located centrally (eccentric)
cartilagenous vertebral end plates
source of nutrients via diffusion
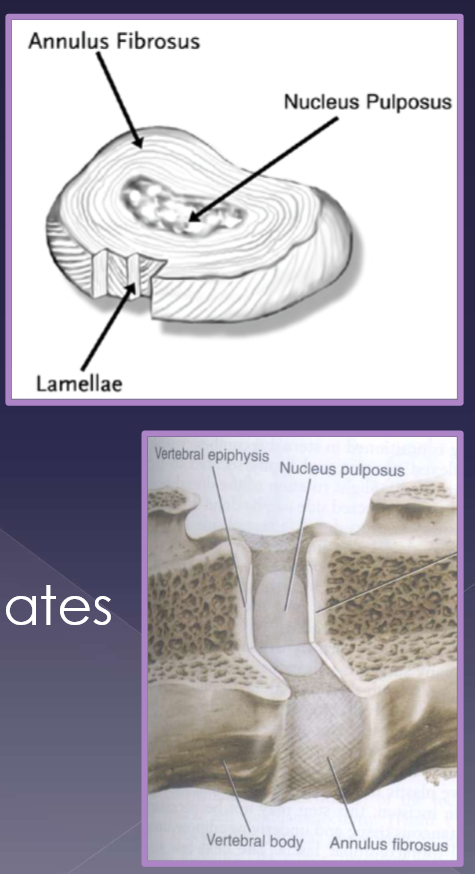
function of the intervertebral disk
shock absorption and distribution
determined by:
proteoglycans in the nucleus
elasticity of the annulus
flexible enough to allow/rigid enough to endure:
bending
shear
torsion
compression
tension
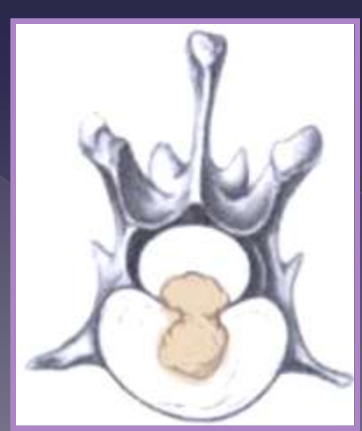
intervertebral disk herniation
chondroid metaplasia
loss of water content
deposition of mineral
alteration of proteoglycans
equals intradiskal pressure
signalment
chondrodystrophic breeds
dachshund - 10x increased risk
3-5yr (TL), 8-12yr (cervical)
clinical presentation
compressive myelopathy
contusion injury
acute, progressive
severity may depend on factors:
location of compression
duration of compression
velocity / force of herniation
volume of disc herniation
clinical presentation for intervertebral disk herniation
paraspinal hyperesthesia
abdominal discomfort
reluctance to ambulate
hunched back
guarded neck
vocalization
forelimb lameness
nerve root signature
ataxia / paresis
paralysis (plegia)

cervical or thoracolumbar intervertebral disk herniation
Cervical
25-33% incidence
C2-3 to C5-6
pain more common
up to 61%
thoracolumbar
66-75% incidence
T10-11 to L6-7
pain + neurologic deficits more common

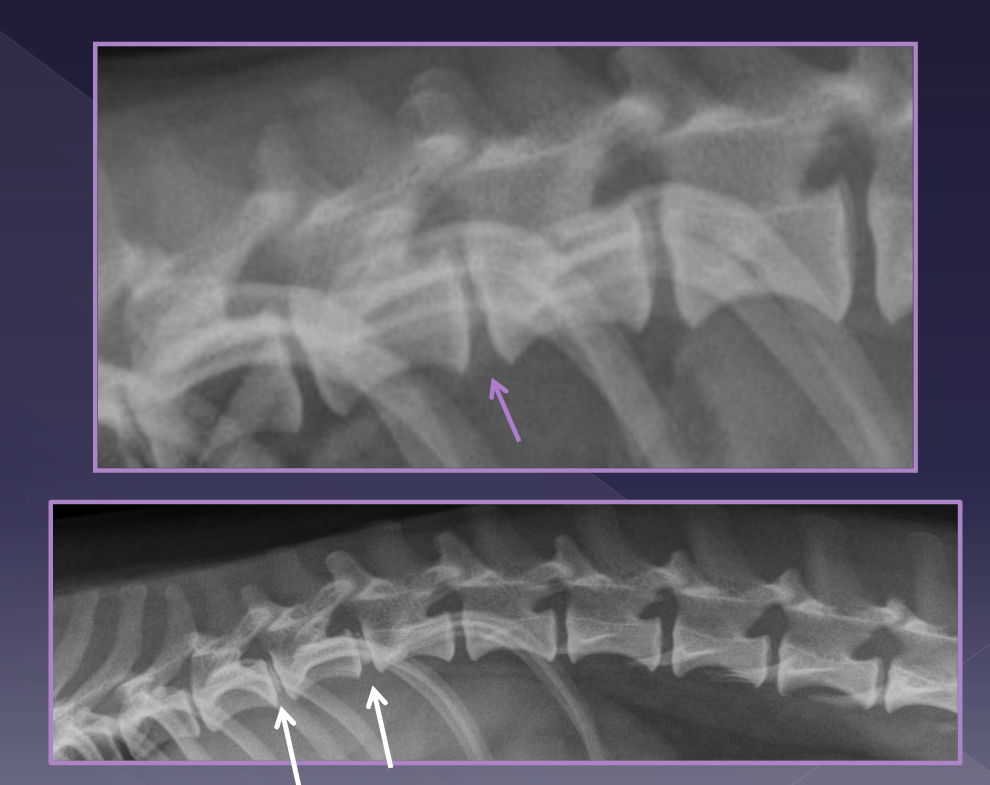
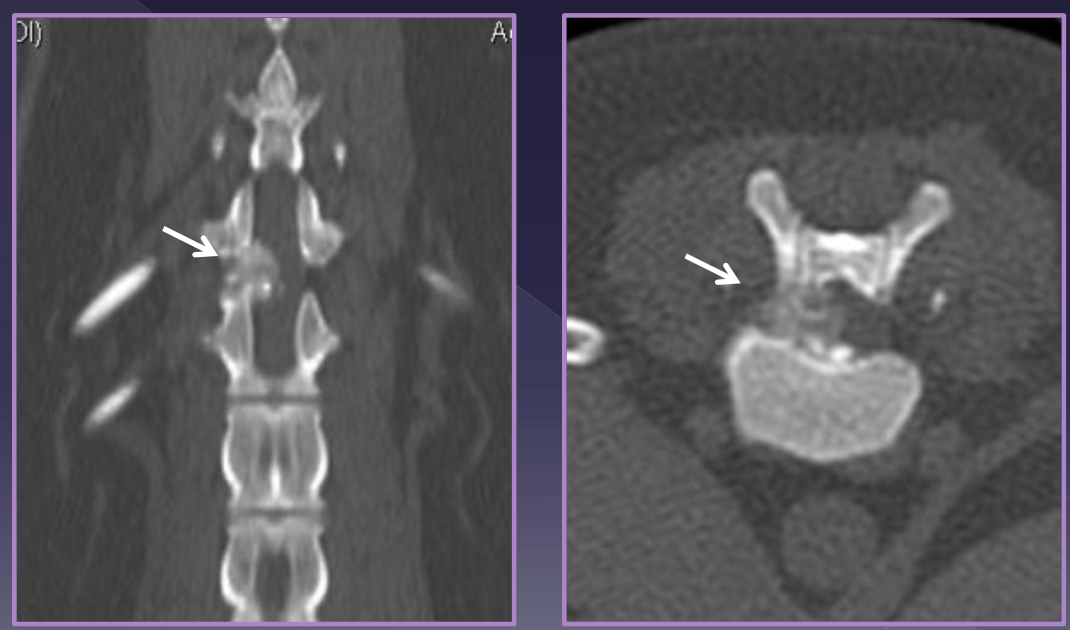
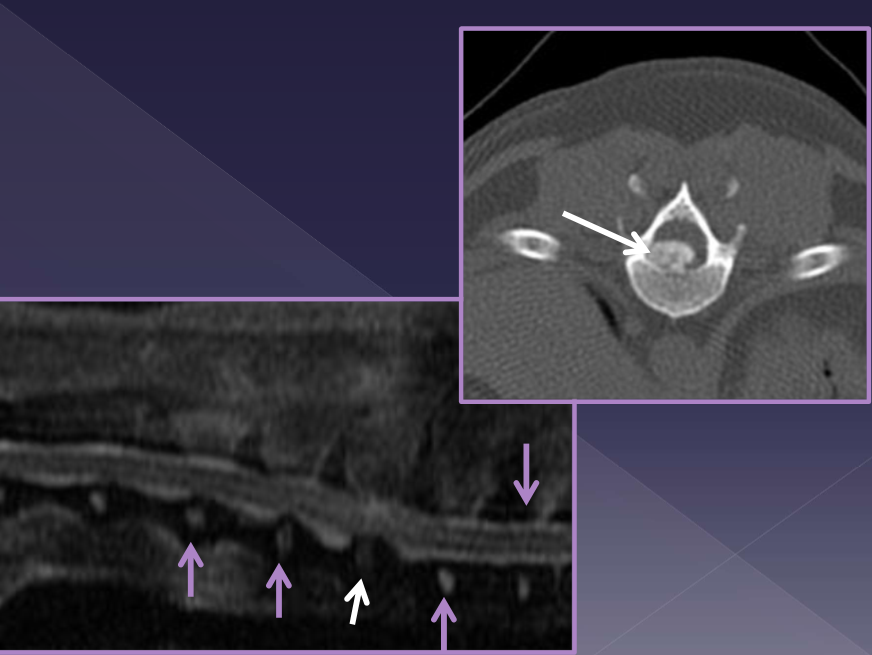
Diagnosis of intervertebral disk herniation
spinal radiographs
35% accurate
rule out differentials
diskospondylitis
neoplasia
trauma
identify
foraminal changes / mineralization
narrowing/wedging of disc space
mineralized disk IN SITU
CT
MRI
management for intervertebral disk herniation
medical management
goals
reduce inflammation
disk resorption
decreased motion
crate rest x 4 weeks minimum
leash walks only
pain medication
Gabapentin - 10-14mg/kg TID
NSAID (carprofen, meloxicam)
± amantadine (4mg/kg SID-BID)
reserved for
pain only
ambulatory paresis
surgical management
goal
remove compression
reduce inflammation/pain
reserved for:
nonambulatory paresis
plegia/paralysis
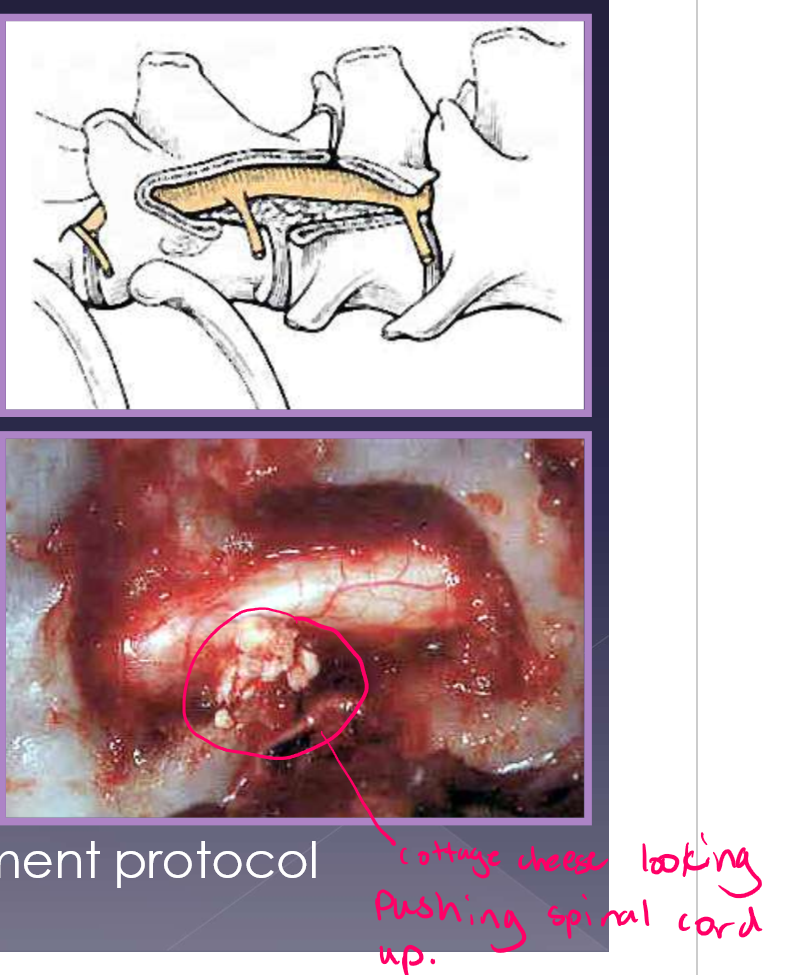
surgical procedure
ventral slot diskectomy (cervical)
hemilaminectomy (TL)
follow with medical management protocol
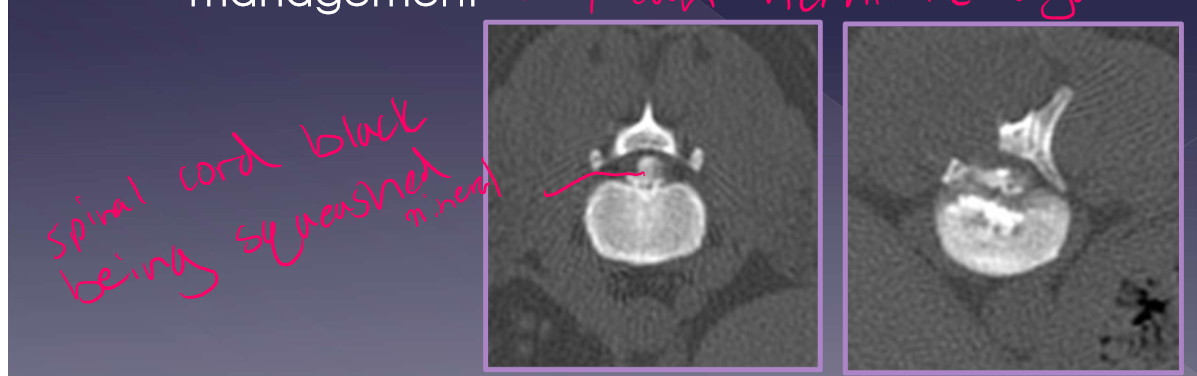
Intervertebral disk herniation recurrence
15-20% - on average but increases with mineralized disk in situ
50% risk with >5 mineralized disk IN SITU
risk increases with inappropriate management - they can herniate again so 4-6 weeks rest important
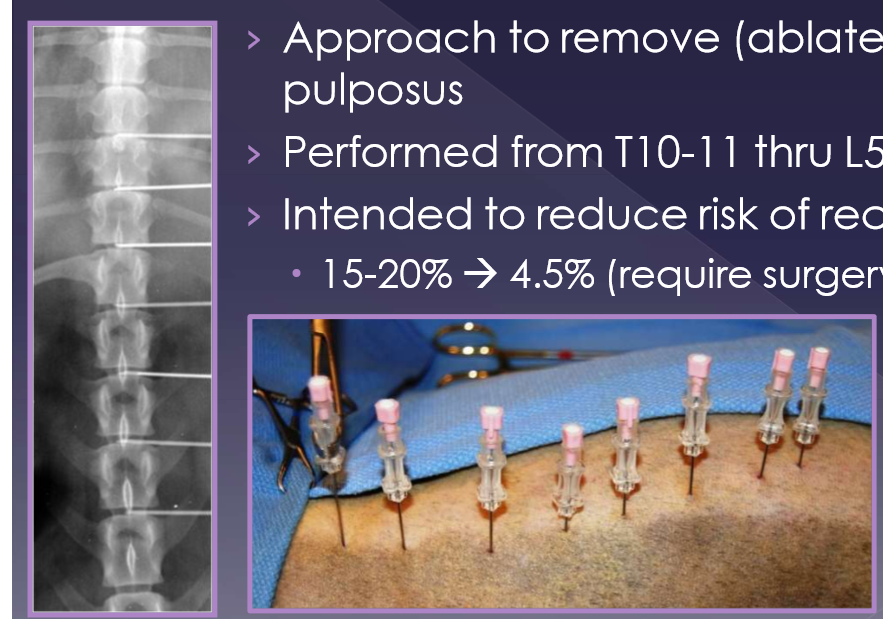
percutaneous laser disk ablation (PLDA) for intervertebral disk herniation
approach to remove (ablate) the nucleus pulposus
performed from T10-11 thru L5-6
intended to reduce risk of recurrence
15-20% → 4.5% (require surgery)
Intervertebral disk protrusion
2nd most common in GP
Fibroid metaplasia
dorsal annulus weakens
nucleus pulposus protrudes into the annulus
effects
ischemic myelopathy
decreased blood circulation
demyelination / axonal degeneration
slow, progressive myelopathy
signalment
large breed
non-chondrodystrophic
middle-aged to older (5-12 yrs)
scuffing toe nails
difficult to get up, way more common in back legs

clinical presentation and diagnosis of intervertebral disk protrusion
slow, progressive ataxia (weakness)
weeks to months
pain at the site of protrusion
TL »» cervical
differential diagnoses
neoplasia
degenerative myelopathy
infection/inflammation
orthopedic disease
diagnosis
radiographs
rule out other differentials
MRI

treatment for intervertebral disk protrusion
medical
often the preferred treatment
pain medication
Gabapentin - 10-15mg/kg TID
NSAID (carprofen, meloxicam)
nursing /supportive care
sling support / ambulatory assistance
padded bedding
good footing
bladder management (if needed)
surgical
goals
decompression
restoration of blood flow
axonal regeneration
procedure
laminectomy
acute worsening of neurologic signs
deterioration of signs within 1 year
continue medical management protocol
you dont really see improvement
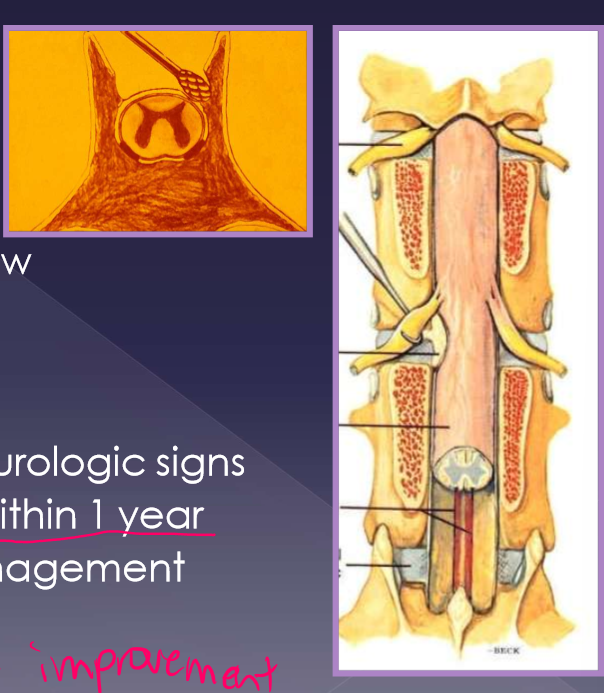
anatomy of lumbosacral disease
L7 to S1-3
collection of nerve roots (cauda equina)
sacrum is attached to the pelvis
joint movement is different
mainly flexion
restricted degrees of lateral bending, rotation, extension
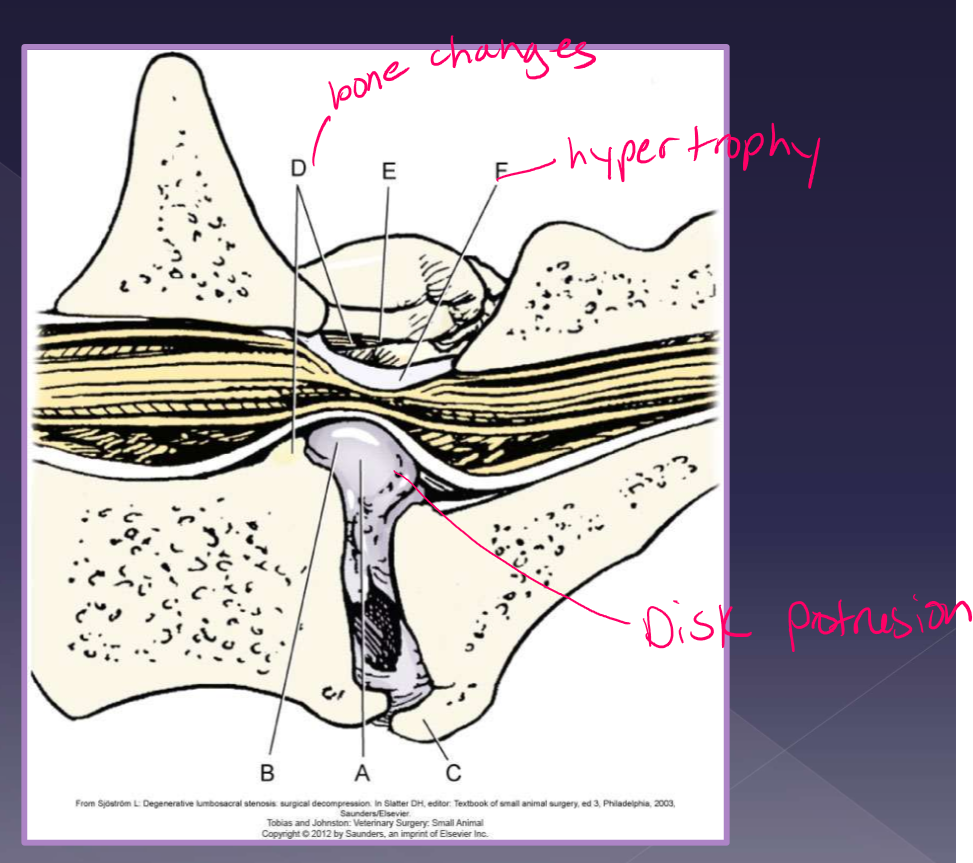
causes of lumbosacral disease
degenerative
disc protrusion* most cases
ligamentum flavum degeneration
dorsal longitudinal ligament hypertrophy
articular facet hypertrophy
spondylosis
instability*
congenital - very rare
stenosis of canal
malarticulation / malformation
transitional vertebrae
osteochondrosis of vertebral end plate
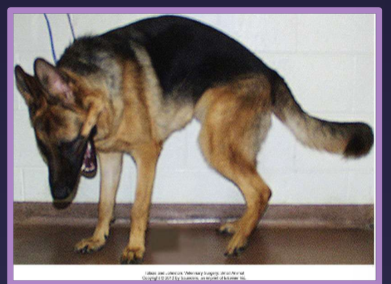
clinical presentation of lumbosacral disease
signalment
large-breed, middle-aged
active, working (German shepherd)
signs
reluctance to jump
less active
stiff hind limb gait - not front legs
low tail carriage - b/c lessens pain so if you lift tail will have pain
urinary or fecal incontinence
lower back pain/ weakness due to vascular compromise and or nerve root compression
pain ± neurologic deficits
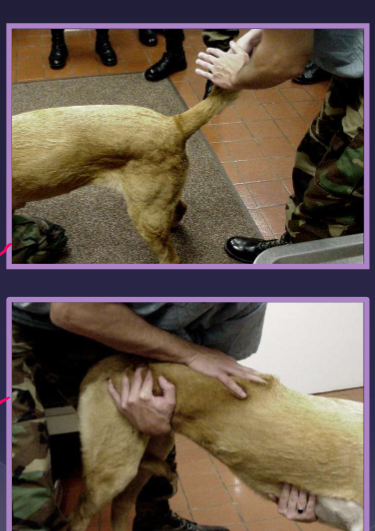
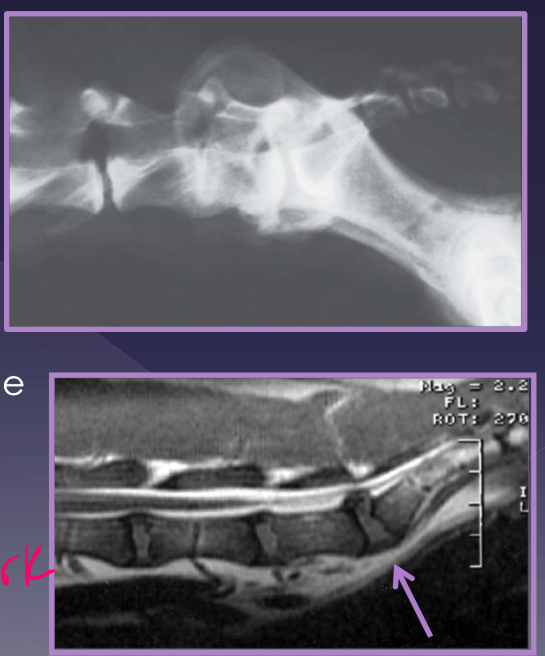
Diagnosis of lumbosacral disease
differentials
neoplasia
hip dysplasia
degenerative myelopathy
elicit pain on examination - tail raise
varying degrees of nerve deficits
sacral, caudal, sciatic nerves
LMN (L4-S3)
abnormal perineal sensation
patellar “pseudohyperreflexia” - exaggerated reflex
sciatic affected, patellar reflex spared - sciatic cant counter the femoral nerve action
due to loss of antagonistic muscle action
femoral nerve not affected since femoral is higher up
rectal exam
palpate dorsal/pressure b/c they will show pain also stricture of rectum
radiographs
spondylosis
narrowed disc
endplate sclerosis
transitional vertebrae
CT
MRI - way to go but CT will work
Treatment of lumbosacral disease
medical
pain medication
gabapentin
NSAID
rest*
epidural steroid injections
methylprednisolone acetate x3 injections
initial 2 weeks, 6 weeks
79% improved; 50% resolved pain
appropriate bedding
bladder control (if needed)
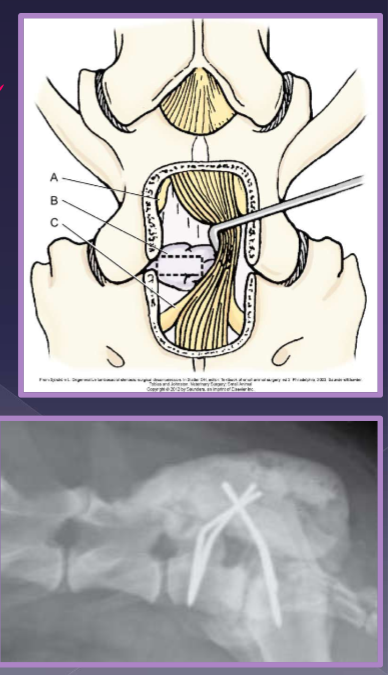
surgical
goal
decompression
pain relief
axonal regeneration
laminectomy + discectomy
distraction and stabilization
prognosis and outcome
laminectomy and discectomy
70-80% success rate
best surgical outcome
mild disease
50% success rate with severe neurologic signs
recurrence
new bone formation
scar tissue formation
wobbler syndrome
cervical malformation or cervical protrusion →
compression of spinal cord / nerve roots →
pain and neurologic deficits
vertebral canal is proportionally smaller
dynamic and static disease
disc-associated vs osseous-associated compression

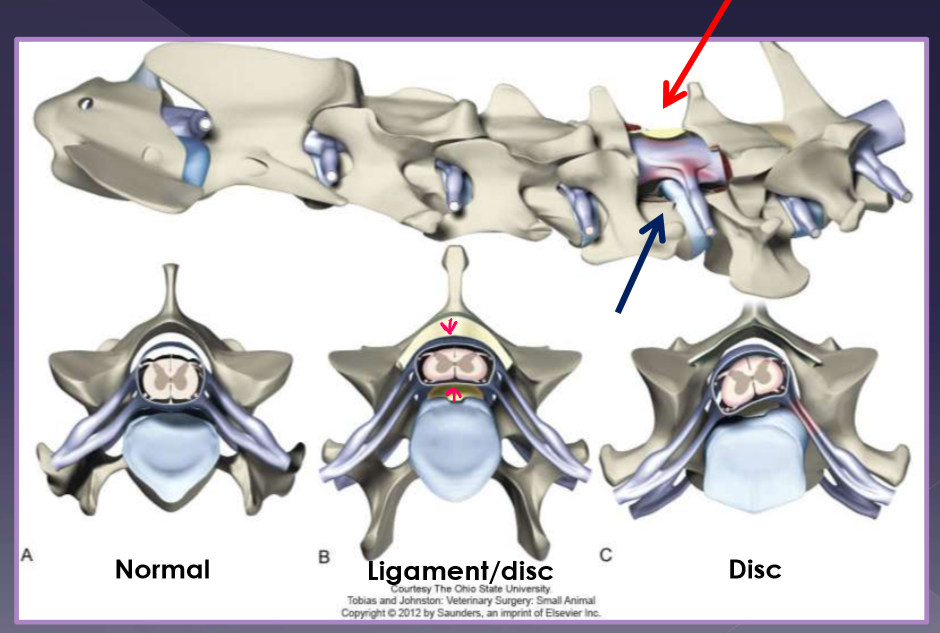
Disc-associated wobbler syndrome
middle-aged, large breed dog
Doberman pinscher*
vertebral canal stenosis (C5-6,6-7)
torsion of the caudal cervical
intervertebral disc protrusion
ligamentum flavum hypertrophy
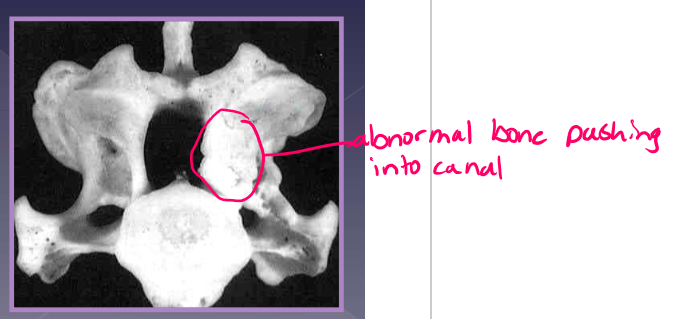
osseous associated wobbler syndrome
young, giant breed, 9 ½ months old
Great Dane*
ligamentum flavum hypertrophy
vertebral canal stenosis due to:
proliferation of the vertebral arch
articular process proliferation
pedicle proliferation
clinical presentation for wobbler syndrome
Chronic, progressive (weeks to months)*
acute presentation - neck pain
mild to moderate proprioceptive deficits / ataxia, lameness
signalment
large breed (3+ years, mean age 7 years)
50% single site / 50% multiple sites
C6-7 most common
giant breed (9mo-2 years)
20% single site / 80% multiple sites
C6-7 most common
diagnosis
radiographs - limited information not helpful
CT -better for bone
traditional method
identify direction of compression
MRI * soft tissue better
gold standard
more accurate at identifying site, severity, nature of compression
assessment of spinal cord parenchyma
sometimes have to do MRI and CT
treatment for wobbler syndrome
medical
reserved for mildly affected cases
exercise restriction
harness instead of neck load*
pain medications - gabapentin, NSAID
good footing, padded bedding
success
54% improved; 27% unchanged neurologically
clinical signs improved or stable - 81%
surgical
indications
more severe neurologic signs and pain
lack of response to medical management
short and long-term expectation of owner
presence of concurrent problems
success
improvement in 81%of cases
techniques for wobbler syndrome and prognosis
direct decompression: static lesions
ventral slot
dorsal laminectomy
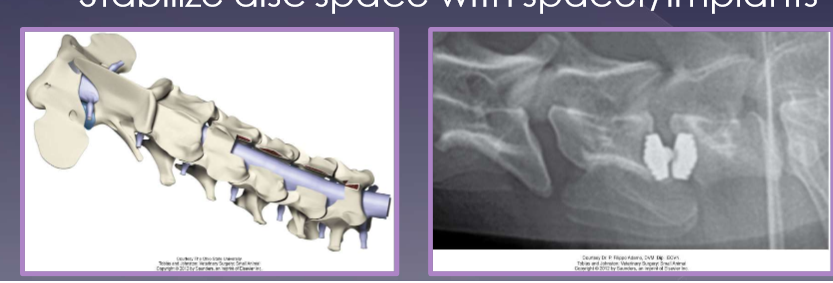
distraction - stabilization: dynamic lesions
decompress disc protrusion
stabilize disc space with spacer / implants
neurologic deterioration
most significant with a continuous laminectomy (70%)
domino effect
adjacent segment syndrome (20%)
mainly with distraction - stabilization techniques
altered biomechanics at operated sited leads to stress/load at adjacent site
up to ~80% improvement
risk of recurrence -25%
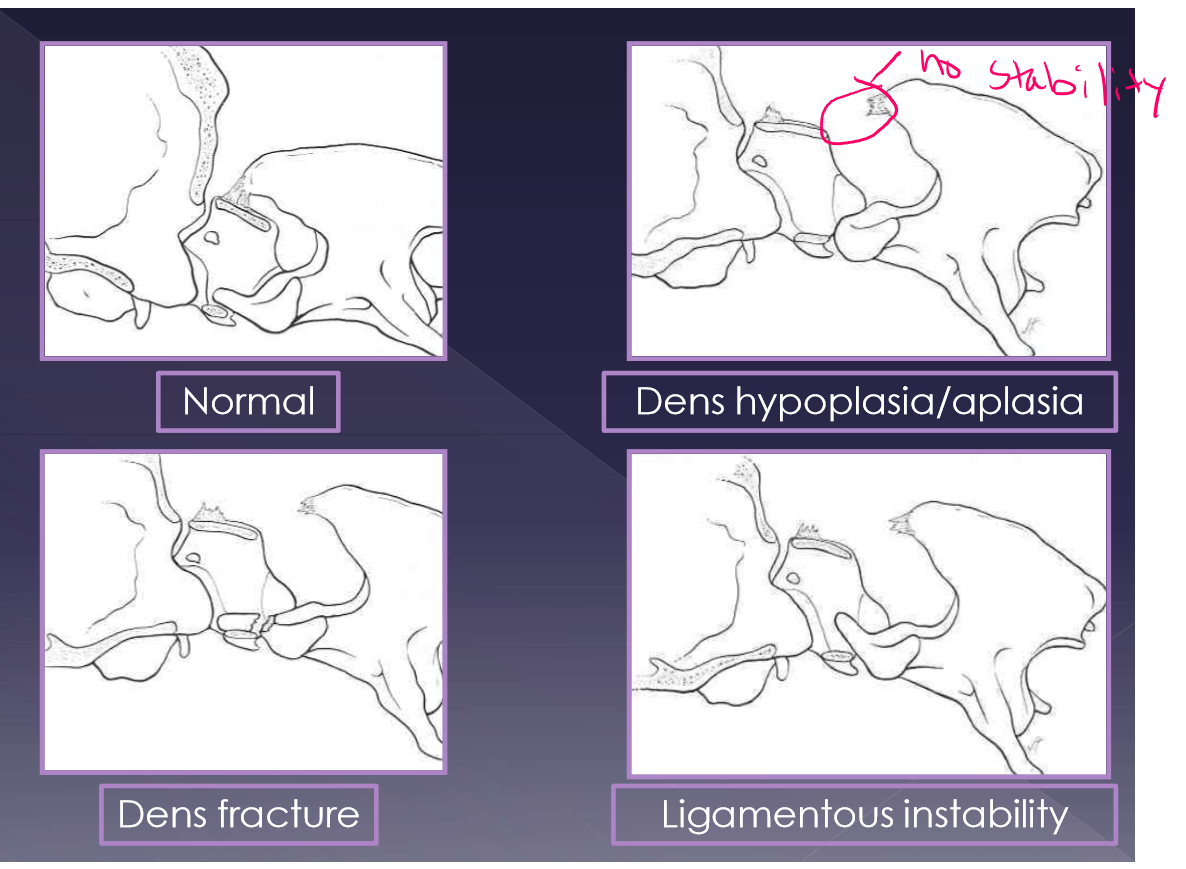
Atlantoaxial instability
compression and concussion of cranial cervical spinal cords
displacement of vertebrae into spinal canal
C2 dorsally displaced from C1
due to:
ligamentous instability
osseous abnormality
congenital or traumatic
effect:
excessive flexion of the A-A joint
cranial axis displaces dorsally in relation to atlas
abnormalities
aplasia of the dens - 46%
hypoplasia of the dens - 34%
dorsal dens angulation
separation of the dens
ligamentous instability
up to 24% of dogs with A-A instability have an normal dens
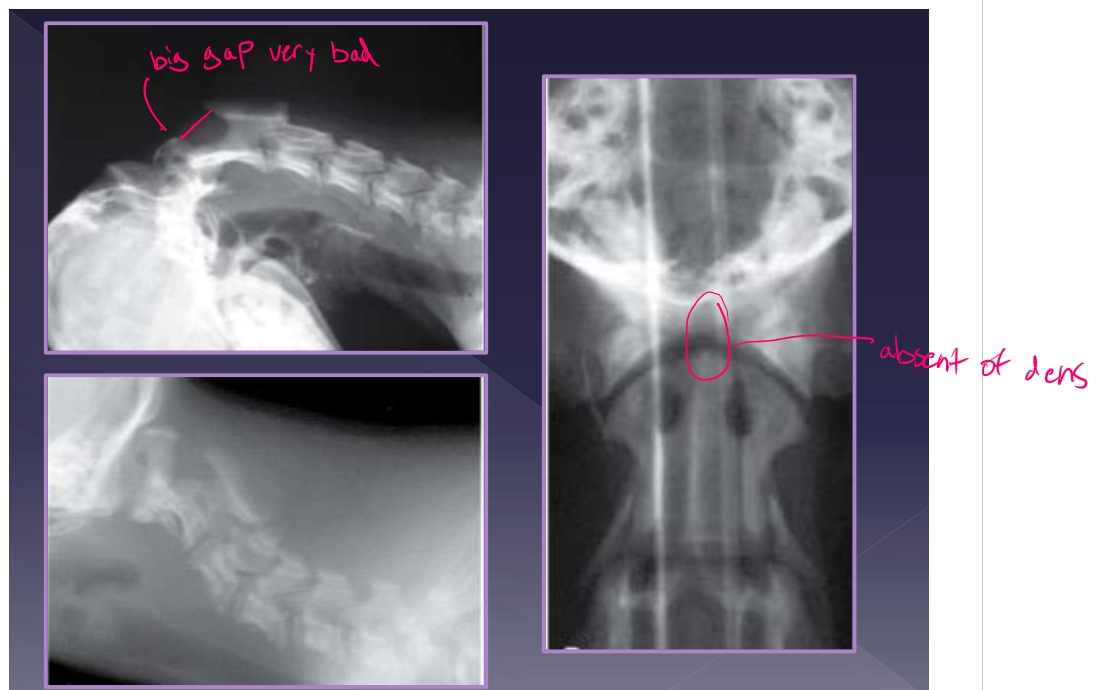
clinical presentation for atlantoaxial instability
young, small breed dogs (<1 year)
toy poodle
yorkshire terrier
chihuahua
severity of signs is dependent on degree of spinal cord injury
clinical signs
neck pain
most traumatic
30-60% congenital
neurologic deficits (94% of patients)
progressive tetraparesis ataxia
diagnosis for atlantoaxial instability
physical examination: Do not flex the neck! makes C2 go dorsally and makes pain worse in flexion of neck
radiographs
increased distance between dorsal arch of C1 and dorsal spinous process of C2
CT
MRI
differential diagnosis
meningitis
syringomyelia
discospondylitis
disc disease

Treatment for atlantoaxial instability
Medical
goal: stabilize joint while ligamentous structures heal
candidates
acute onset of clinical signs without prior history
young dog with immature bone
financial constraints
strict confinement: 6-8 weeks * very important
neck brace*
pain medications
Gabapentin, NSAID
good long term outcome
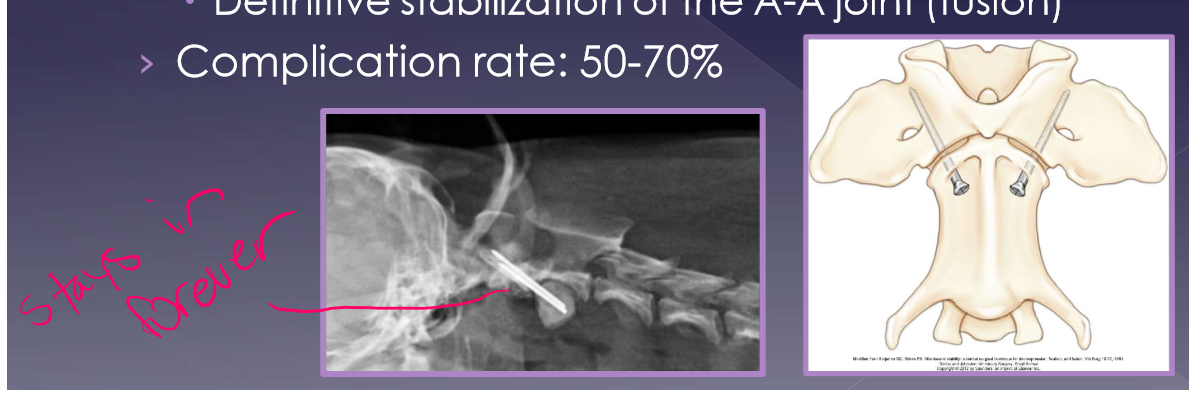
surgical
gold standard
goal
reduce further compression of the spinal cord
definitive stabilization of the A-A joint (fusion)
complication rate: 50-70%
prognosis
medical management
good long term prognosis in 40% of cases
better if clinical signs <30days
perioperative mortality - 10-30%
principles of integumentary system surgery
tissue trauma
scalpel < scissors < CO2 < electroscalpel
skin hooks or suture stays < tissue forceps or repeated tissue manipulation
to decrease the risk of devascularization
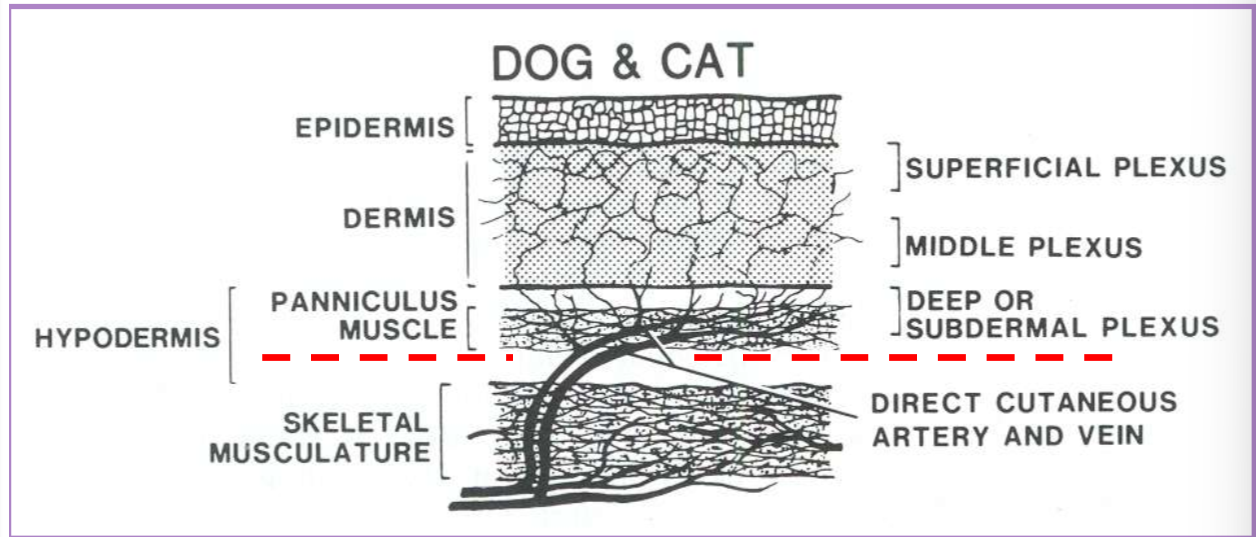
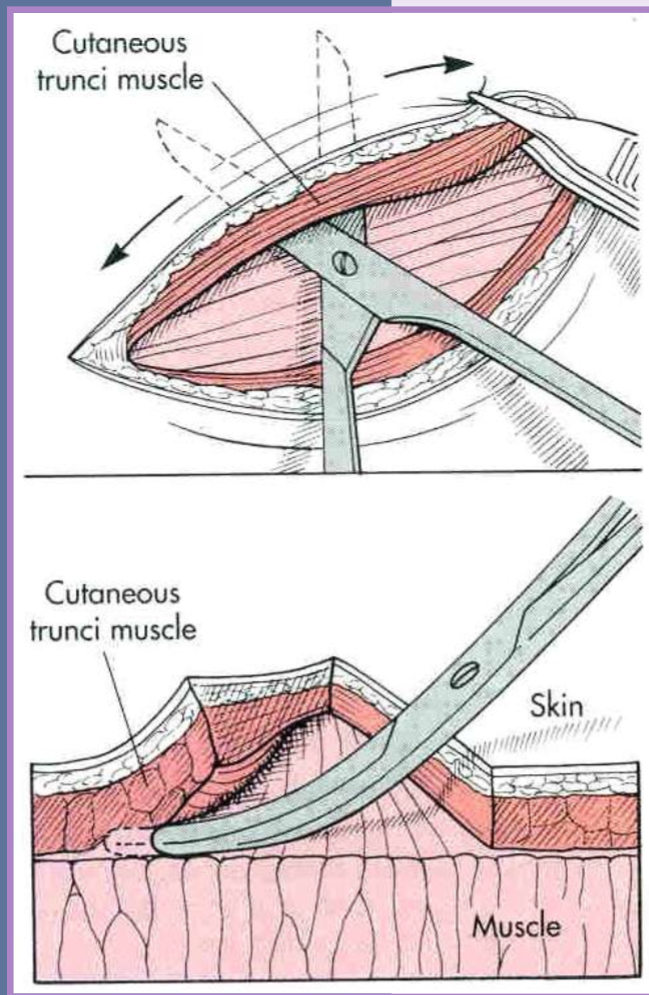
dissect deep to the subdermal plexus
under cutaneous muscles or the deep dermal layer
skin closure
primary
immediate direct closure
delayed primary (<3-5 days)
delayed direct closure before onset of granulation tissue
secondary (>3-5 days)
delayed direct closure after onset of granulation tissue
second intention
indirect closure by onset of granulation tissue and epithelization
technique chosen for skin closure depends on
elasticity of surrounding tissue
location of the defect
size of defect
regional blood supply
wound healing factors - systemic and local
simple is best
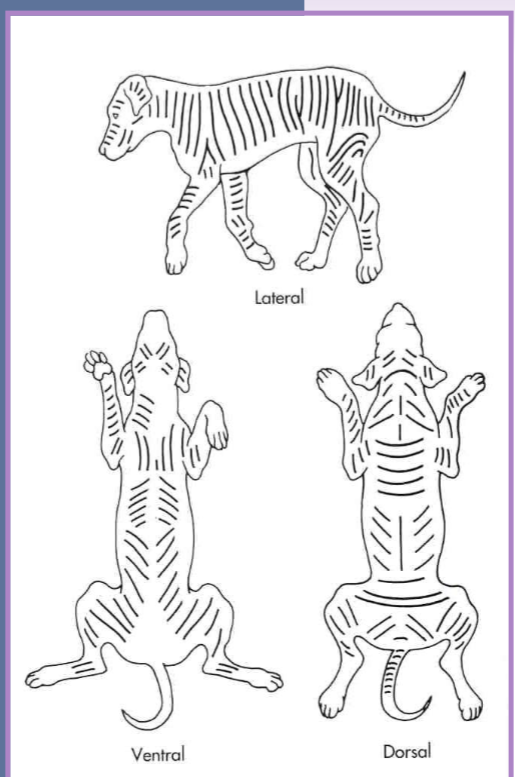
skin tension
tension lines are formed by the predominant pull of fibrous tissue within the skin
tension increases the risk of closure complications
generally close skin parallel to tension lines
if closed perpendicular to skin tension lines:
delays in healing
wider scar
more tension - dehiscence, pain
dog ears
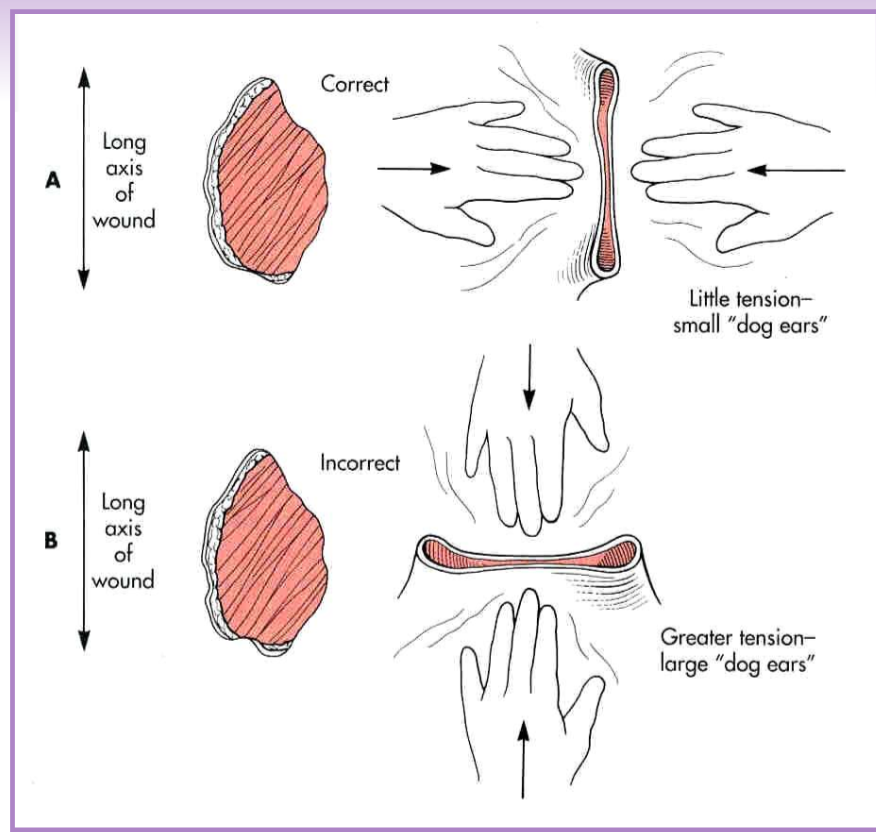
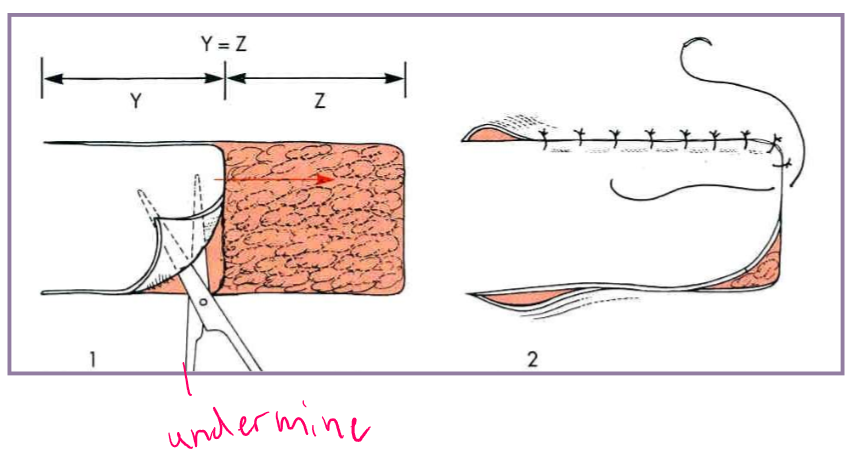
Tension relief - Undermining
separate the skin and panniculus muscle (when present) from underlying subcutaneous tissue
blunt and sharp scissor dissection
simplest technique to relieve tension
maximizes elastic potential of skin edges
undermining - delayed wound closure
separate granulation tissue from epithelium
caution!! dont get too aggressive
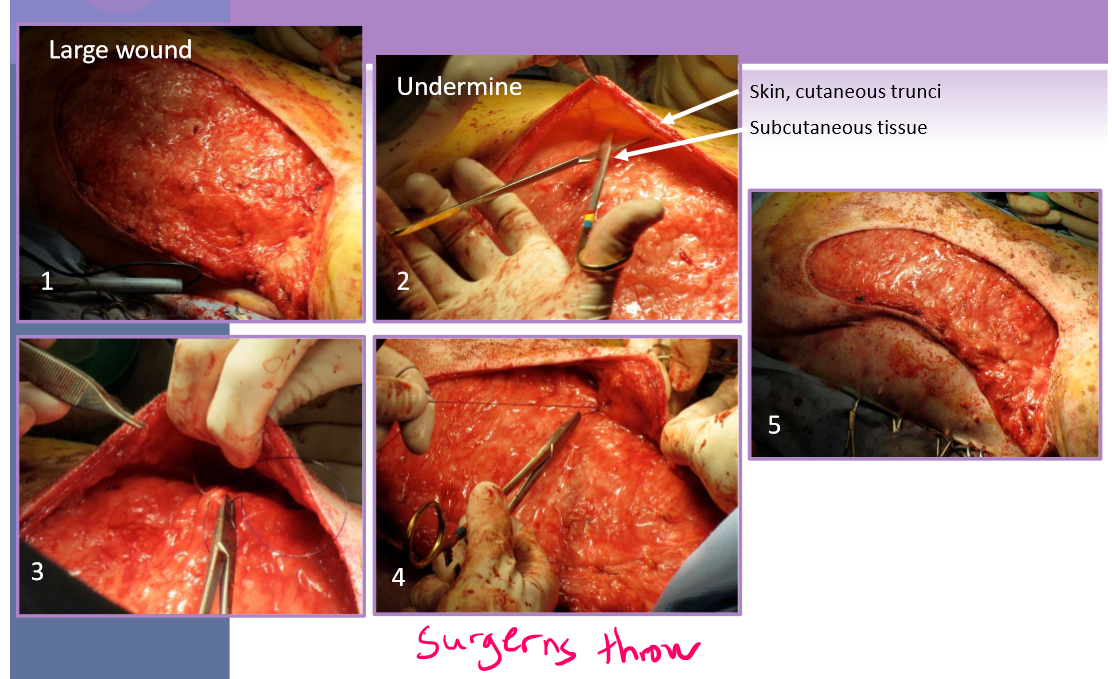
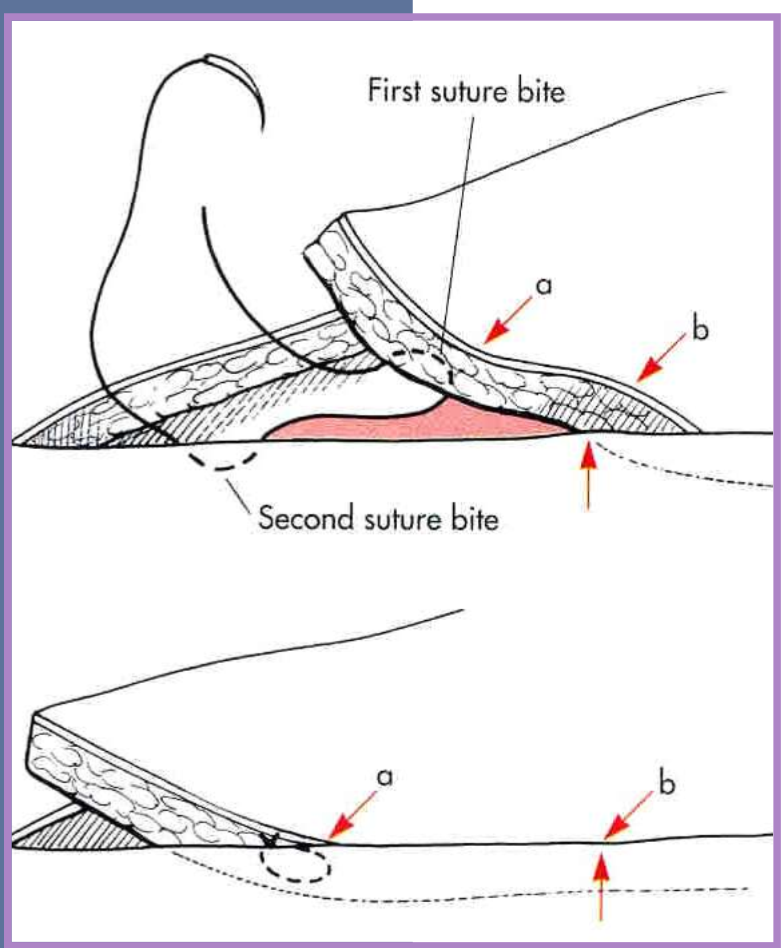
Tension relief - walking sutures
must do undermining first
moves skin across a defect
obliterates dead space
distributes tension over the wound surface
via several suture rows
less chance of dehiscence of main incision closure
stretches skin in small increments
anchored in fascia and dermis
do not penetrate the skin surface
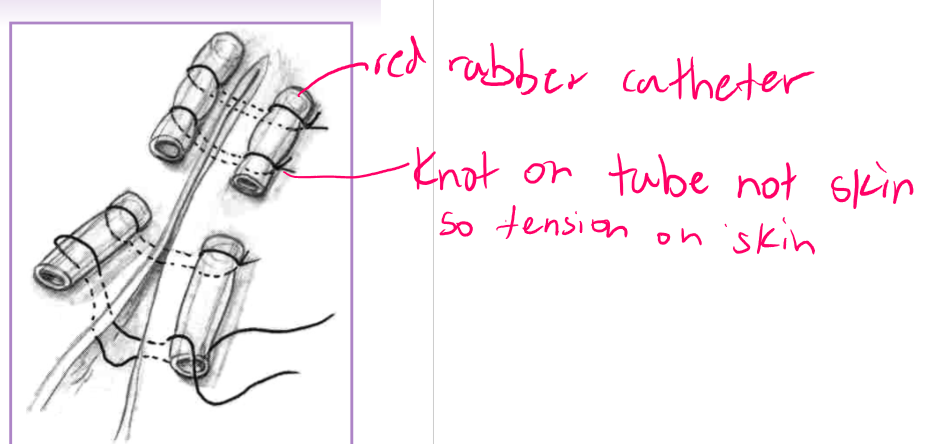
tension relief - suture patterns
cruciate
horizontal mattress
vertical mattress
far-near-near-far
help prevent sutures from cutting out
provides limited tension relief
can place sutures farther from skin edge or use suture pattern to help disperse pressure
cruciate - in skin
horizontal / vertical mattress - in fascia/deep tissue
far-near-near-far
stents and quills
skin stretching
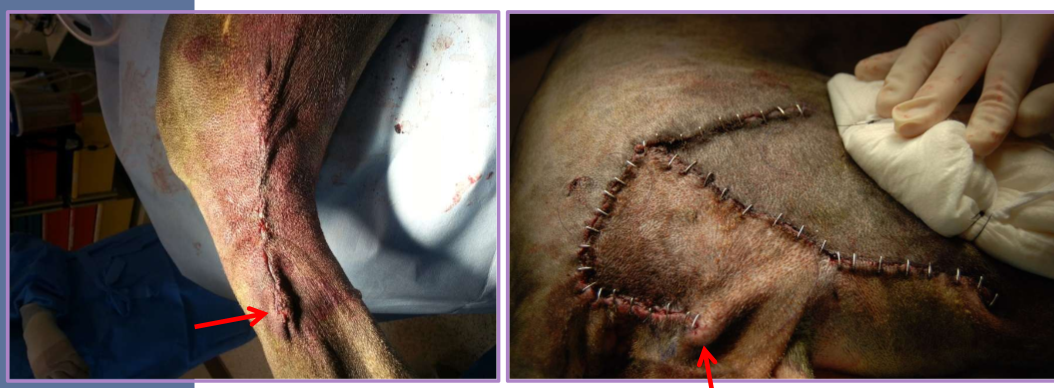
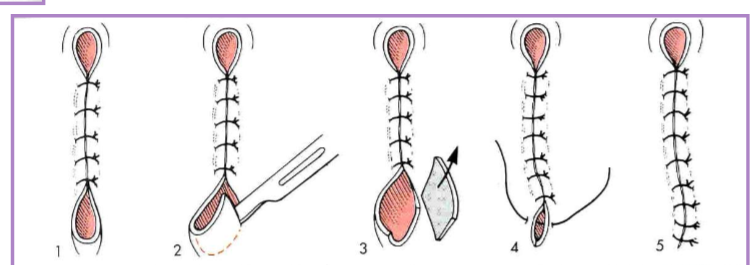
Dog ears
puckers of skin at the end of the incision line
correction via various methods
outline with an elliptical incision, remove redundant skin, appose skin edges
cut off the dog ear - blade or scissors
irregular circular skin defects
difficult to close because of dog ears
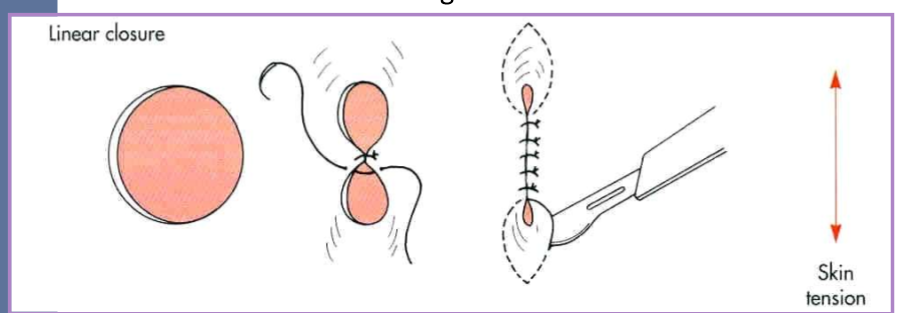
perform a linear closure
easier with smaller defects
close parallel with line of tension
* start in the center of the wound
correct the dog ears
convert to ellipse
easier with smaller defects as well
resects more skin than necessary
4:1 length to width ratio
eliminates dog ears
adequate surgical preparation
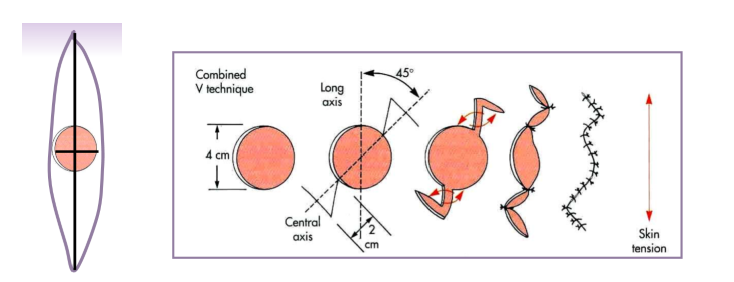
combined V - good with lesion by eye
45 degrees from axis of tension
additional skin is not removed
triangular defects in skin
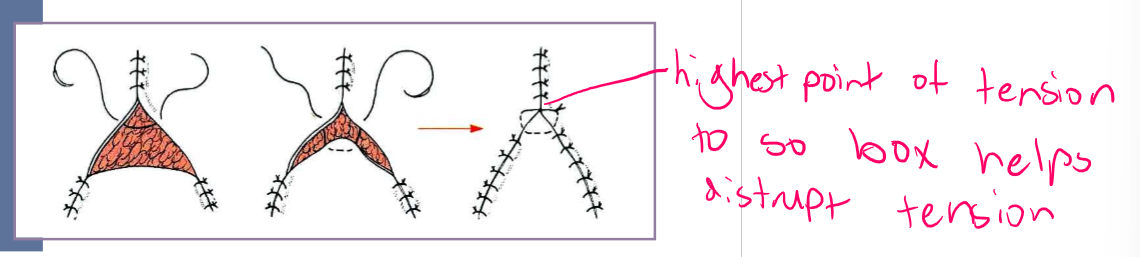
Y closure
start at points of triangle and suture towards center
place horizontal type suture in center (where most tension is)
rotational flaps
semicircular / three quarter circular flap of skin rotated at a pivot point into the defect
single
used when skin is available only on one side of the defect or rotation of skin results in a defect/distortion of adjacent structures
bilateral
little skin is available on both sides of defect
4:1 length to width - prevent tension
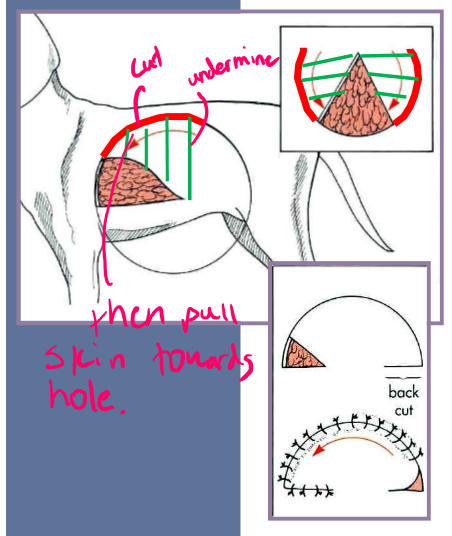
irregular skin defects
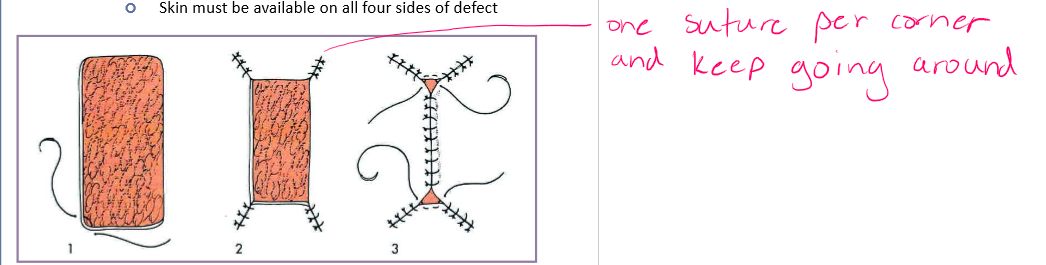
square or rectangle
start at corners and work inward
skin must be available on all four sides of defect
advancement flaps
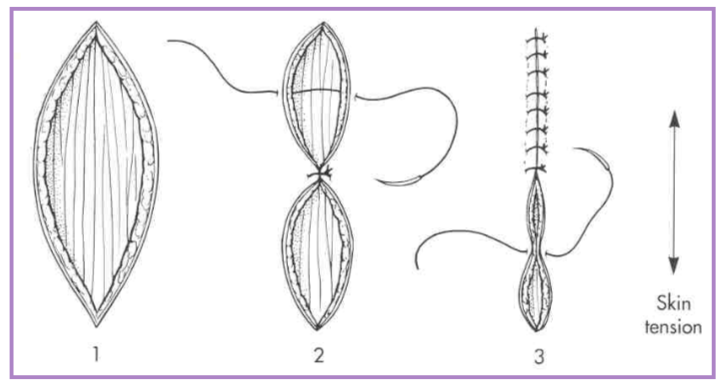
fusiform (elliptical) skin defects
place suture across widest part of defect (center)
divide each remaining segment in half with subsequent sutures
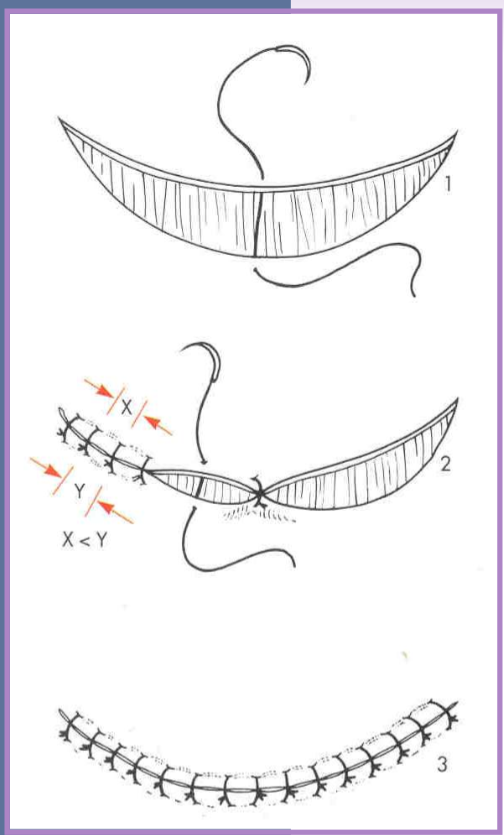
crescentic skin defects
one side is longer than the other
close beginning at the midpoint
each remaining segment closed by dividing segments in half
place sutures on concave side closer, further on convex side
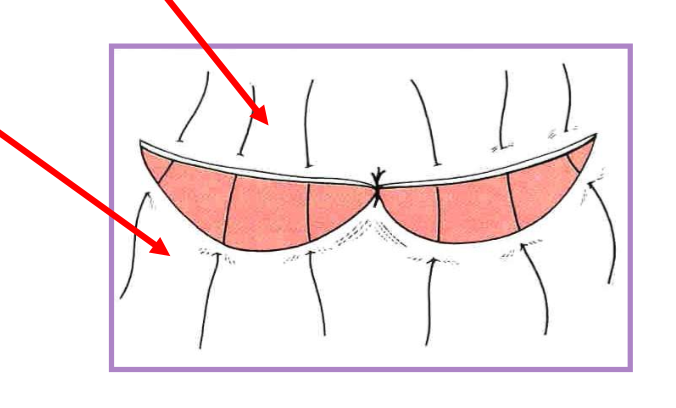
skin grafts
transfer of a segment of free dermis and epidermis to a distant recipient site
full thickness (recommended)*
epidermis and dermis
partial thickness
epidermis and variable dermis - part
cannot do in cats (too thin)
types
sheet or mesh (often preferred)
punch, stamp, stripe
mesh preferred b/c
increased surface area
better conformity
fluid drainage
factors critical to graft survival
healthy vascular bed - no infection
lack of motion
contact between the bed and graft
lack of infection
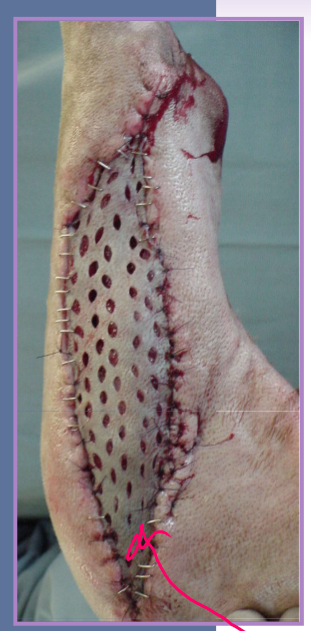
Neoplastic masses
wide excision
submit tissue for histopathologic margins
first attempt at removal is the best attempt
exact margin depends on tumor type
lipoma - 0cm
mast cell tumor - 1-2cm
high grade sarcoma - 3cm
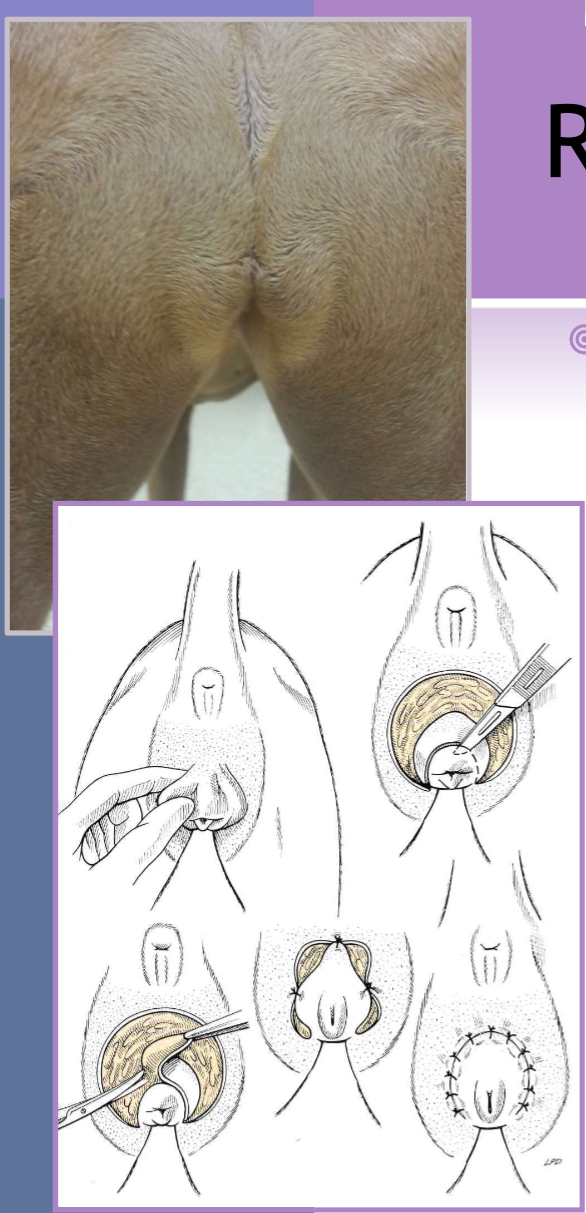
vulvar fold redundant skin
signalment
overweight dogs
younger dogs with an infantile, recessed vulva * most commonly seen
superficial dermatitis, urinary incontinence, recurrent urinary tract infections
episioplasty
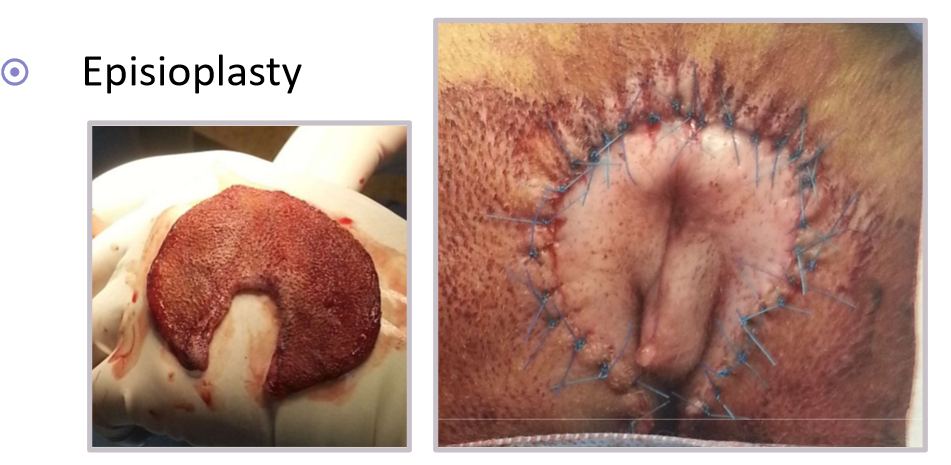
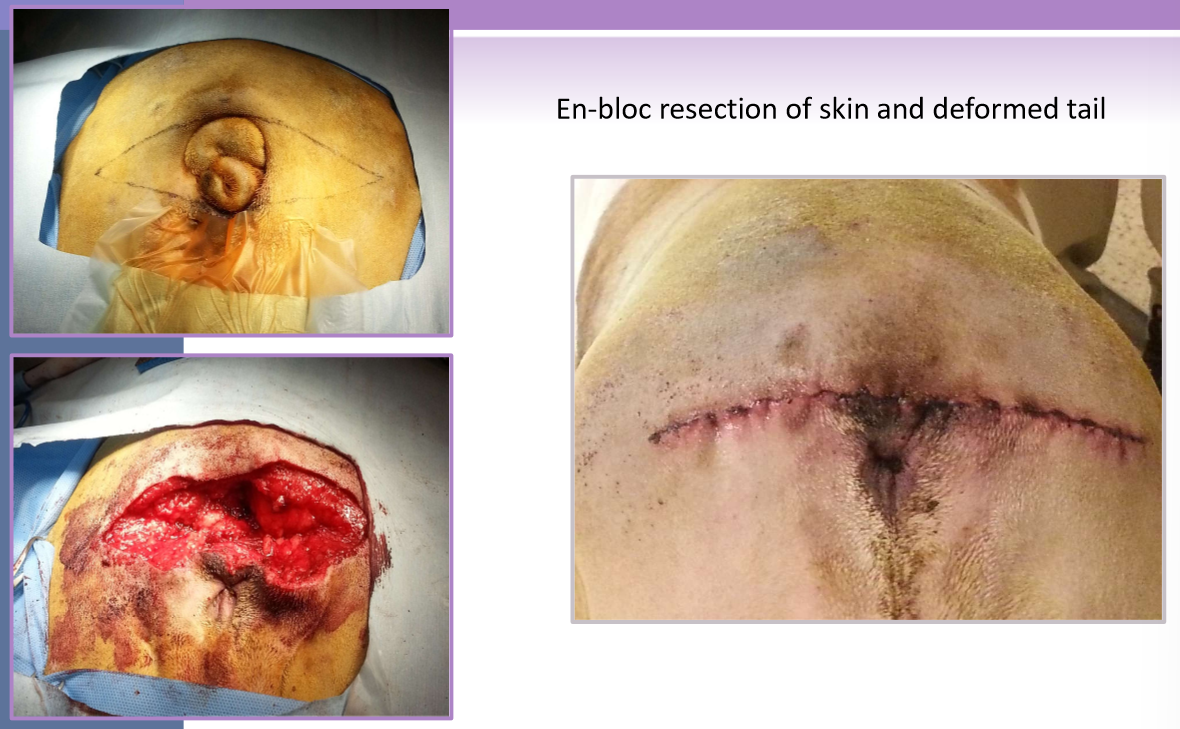
redundant tail folds
signalment
Brachycephalic
bulldog, Schipperke, Manx cat
screw tail, corkscrew tail, ingrown tail
redundant skin overlaps a deformed terminal caudal vertebrae
tail fold pyoderma secondary complication
en-bloc resection of skin and deformed tail
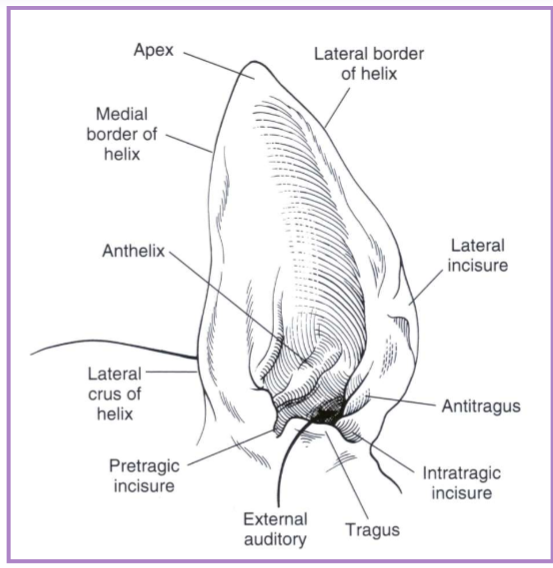
pinna lacerations
lacerations
skin
skin + cartilage
skin + cartilage + skin
use auricular cartilage as a template
partial thickness may be treated conservatively
marginal defects may be amputated
Pinna aural hematoma
collection of blood, serum, or both in the pinna
forms second degree longitudinal planar fracture of articular cartilage
exacerbated with shaking of the head
numerous techniques for repair
goal: obliterate dead space
incision and suture, CO2 laser
infusion of steroid
common with ear infections shaking head
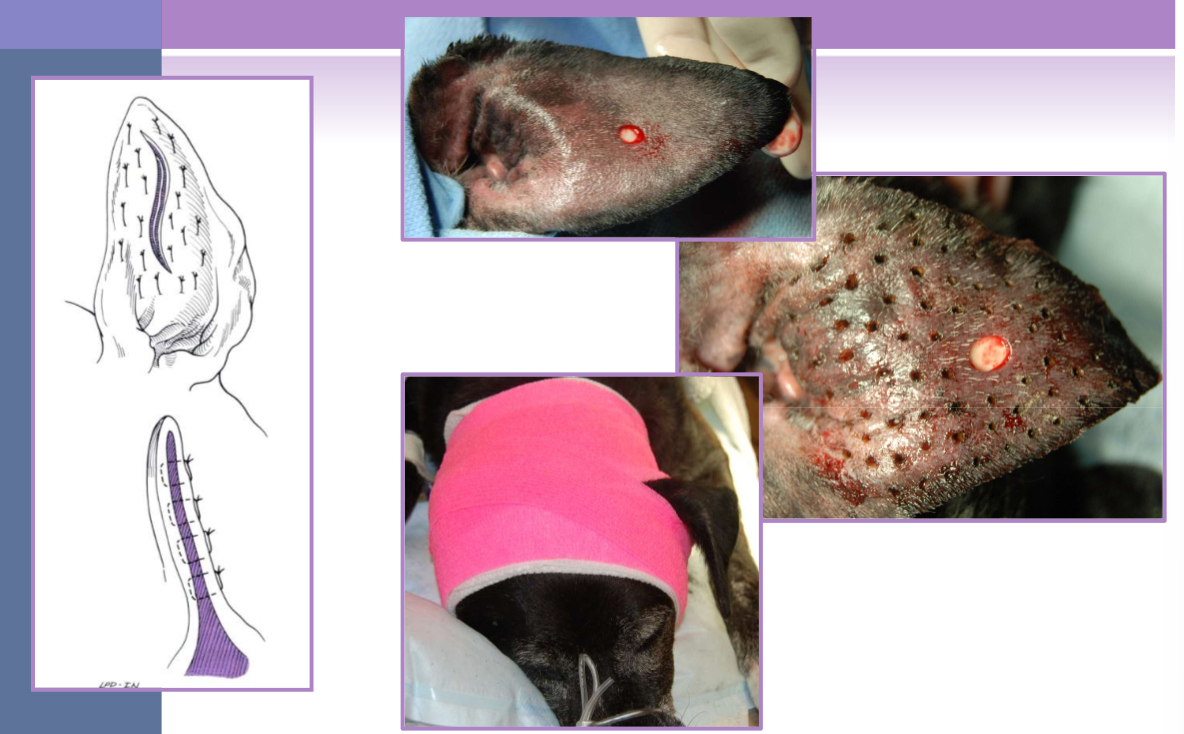
lateral ear canal resection for otitis externa / media
removes the lateral wall of the vertical ear canal
new opening is at junction of vertical and horizontal canal
indications
adjunct to medical management for otitis externa
minimal hyperplasia of ear canal
small neoplastic lesions of lateral aspect of vertical canal
allows for increased drainage, aeration of canal, facilitates medication administration
owner beware - must still medicate the ear
not for concurrent otitis media or obstruction of the horizontal ear canal
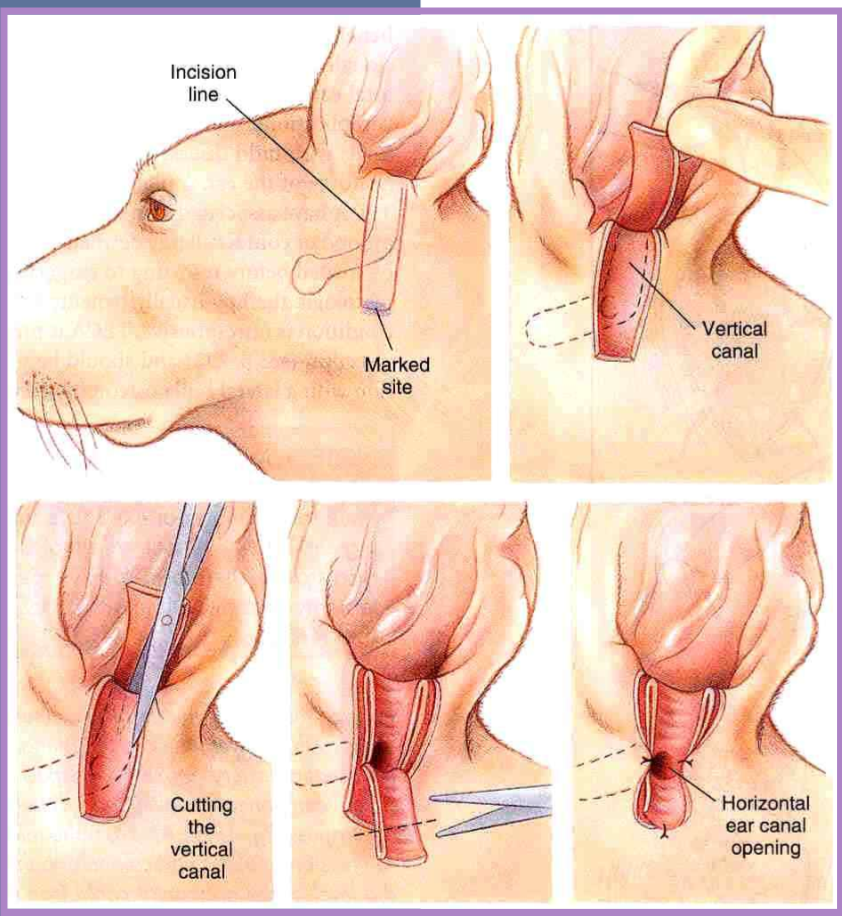
vertical ear canal resection for otitis externa / media
indicated for vertical canal disease
tumor infiltrating vertical canal
otitis externa confined to the vertical ear canal
horizontal canal must be normal
better cosmetic appearance than with lateral resection when abundant hyperplastic tissue is present in and around vertical ear canal
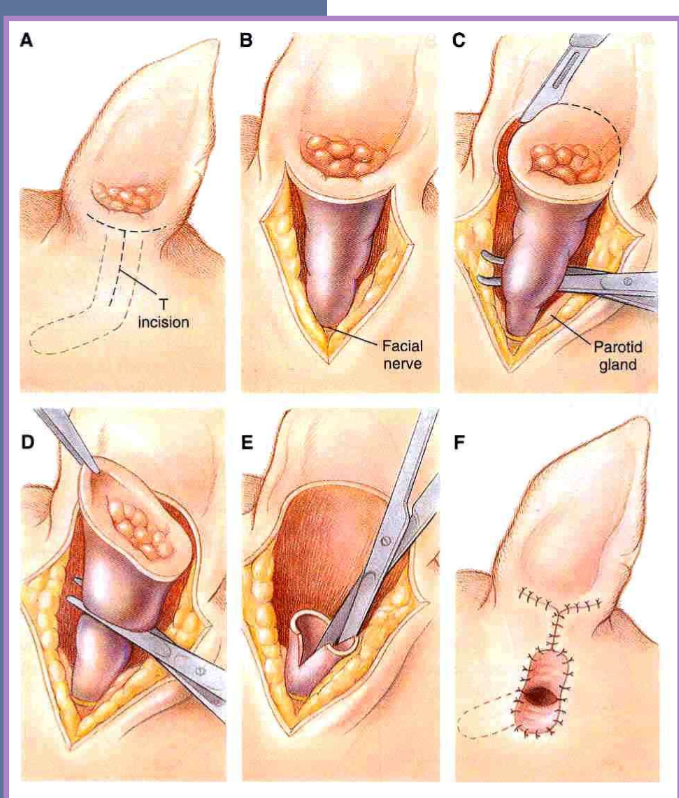
total ear canal ablation (TECA) for otitis externa / media
most commonly performed
indications
chronic otitis externa
failure of medical management
ossification, hyperplasia of entire canal
neoplasia
must perform a lateral bulla osteotomy ( scraping of)
must remove all epithelium in bulla
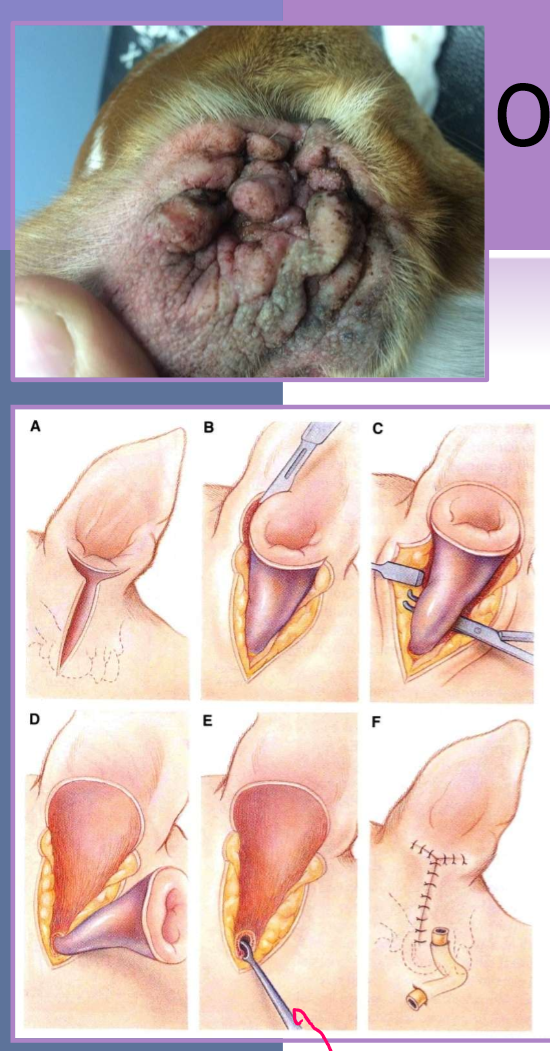
ventral bulla osteotomy otitis media
allows increased exposure to tympanic cavity
technique of choice for cats with:
neoplasia
nasopharyngeal polyps
better drainage of bulla
cats have two compartments that make up their bulla
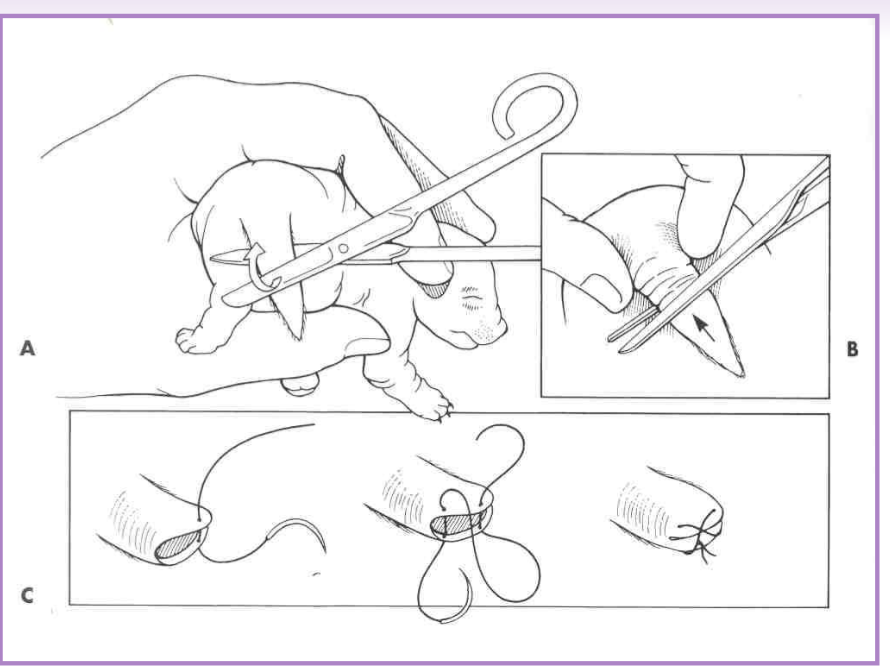
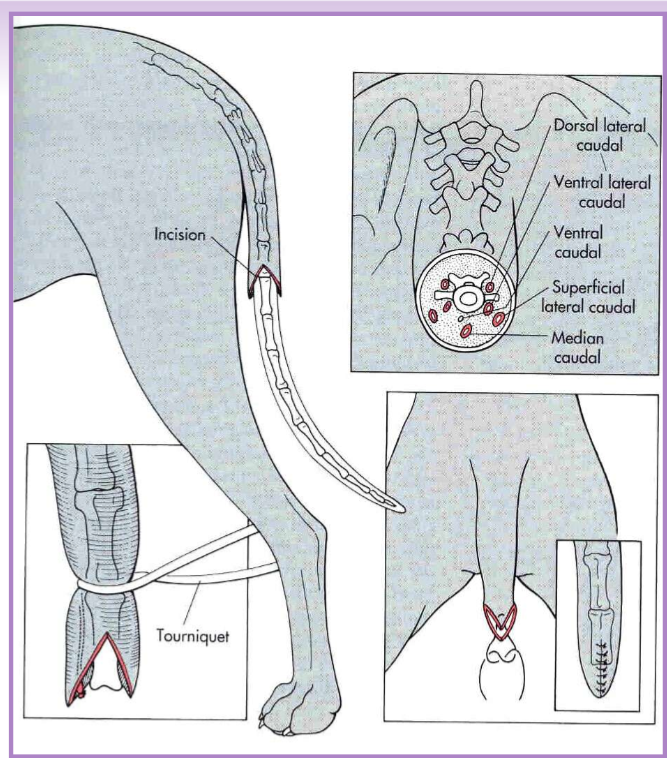
Caudectomy
amputation of a portion / all of the tail
therapeutic caudectomy
traumatic lesions, infection, neoplasia
cosmetic caudectomy - puppies
3-5 days of age
analgesia - usually local
adult caudectomy
general anesthesia
V-shaped incision, vessel ligation, tension free closure
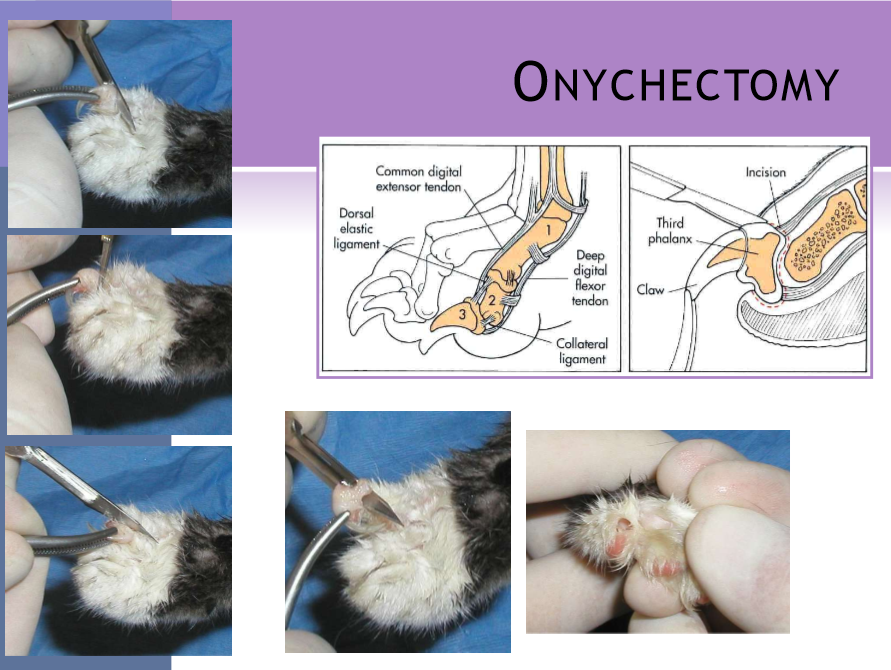
onychectomy
cats
removal of the 3rd digital phalanx (P3)
complication rate ~20%
elective - usually performed between 3-12 mo
young tolerate the procedure better than old
front or all four limbs educate owners
alternatives : nail trim, caps, outdoor cat
surgical procedure
local / ring block to provide additional analgesia
place a tourniquet
radial neuropathy
limit time
scalpel
all of P3 is removed by surgical dissection
resco nail clipper
better chance of leaving ungual crest
can get persistent pain/lameness from remaining bone
CO2 laser
less bleeding
faster healing
smaller incision
disadvantage; laser is expensive
sutures
absorbable, monofilament
single for skin apposition
postoperative care
bandages x 24 hours
paper litter
complications
hemorrhage, infection
claw re-growth, lameness from remaining bone
pad injury, tissue necrosis
onychectomy for dogs - Neuter
indications
trauma
tumor - SCC, melanoma, sarcoma
infection