Bio 111 multiple choice questions
1/37
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
38 Terms
Which of the following is the largest in the hierarchy of organisms to ecosystems?
landscapes
Evolution is the genetic change in what over time?
populations
The structural, physiological, or behavioral traits that enhance an organism’s chances of survival and reproduction are known as
adaptations
What is a scientific hypothesis?
A tentative answer to a question from which testable predications can be made
A researcher is studying the effect of exercise on weight. They set up an experiment where half of the mice are housed with a running wheel and the other half are housed without one. After six weeks, the researcher measures the weight of the mice. Which of the following statements best identifies the predicament in this experiment?
Mice with access to a running wheel will weigh less than those without one after six weeks
which of the subatomic particles have a negative charge
electrons
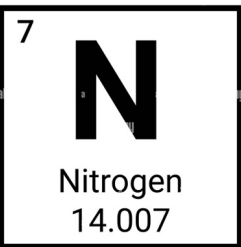
what is the atomic number for nitrogen
7
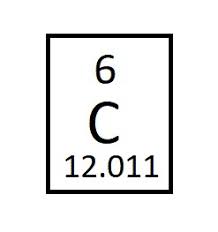
whatt is the atomic mass for carbon
12
how many total electrons are shared between two atoms that have a single covalent bond
2
in a covalent bond, electrons are ______
shared
is C-H a polar or non-polar covalent bond
non-polar
is C-O a polar or non-polar covalent bond
polar
based on its number of electrons and the octet rule, how many bonds does Oxygen make with other atoms
2
when energy is released by a chemical reaction, what happens to entropy
entropy increases
what kinds of chemical reactions produce more energy than they require
exergonic
which of the following would SLOW the rate of a chemical reaction
increase the activation energy
what type of general reaction breaks polymers into monomers
hydrolysis
which of the following is NOT a property of water
low specific heat
you have a solution with a pH of 4, and want it to have a pH of 8. What do you do?
add a base
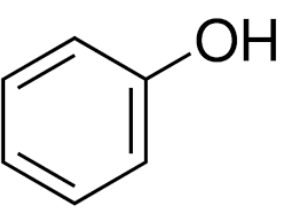
how many hydrogen molecules are depicted on the following diagram
6
what is the monomer of lipids
there are none
which of the following is a defining characteristic of lipids
insolubility in water
what is a consequence of a lipid having a higher melting point
it is solid at room temp.
why do unsaturated fatty acids have a lower melting point than saturated fatty acids
double bonded carbons prevent molecules from packing closely
what does “amphipathic” mean
both water “fearing”, and water “loving”
what is the general biological function of steroids in living organisms
to act as a metabolic messenger in circulatory systems
what is the monomer of proteins
amino acids
what kind of bond would link together the monosaccharide within an oligosaccharide
glycosidic bond
what is the monomer of a nucleic acid
nucleotides
which of the following is NOT a main component of a nucleotide
glycerol
which of the following exists solely as a single-stranded molecule
RNA
According to complementary base pairing rules, what base pairs with guanine
cytosine
According to the central dogma, what is the basic unit of hereditary
DNA
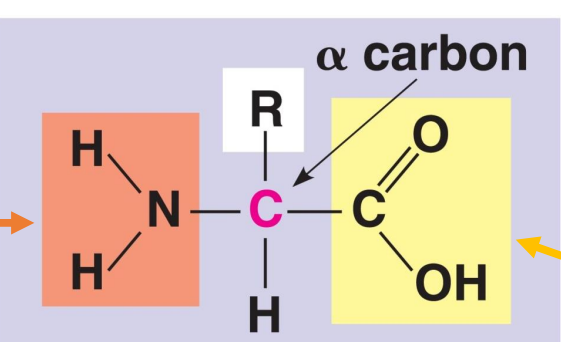
in this general diagram of an amino acid, which is the amino group
NH2
which of the following protein structures is the basic sequence of amino acids, linked by covalent peptide bonds
primary
which of the following protein structures is folded into a 3D shape due to different bonds
tertiary
what does denaturing do
disrupts weak bonds within proteins