7. large animal med- blood gas
1/56
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
57 Terms
what is a blood gas?
portable and relatively inexpensive, rapid assessment of lots of information:
-acid-base
-electrolytes
-lactate
-glucose
-PCV/TP should be run along with blood gas
why are blood gases ran?
-rapid, quick assessment
-provides diagnosis/prognosis information
-detection of life-threatening derangements
-most sensitive indicator of respiratory function
-serial monitoring in hospital
when are blood gases ran?
emergencies
how do you run a blood gas?
step 1: figure out what type of sample is needed (arterial vs venous)
step 2: figure out where to collect sample
step 3: prep (esp. for arterial sticks), poke, and run
when are blood gases ran on venous vs arterial blood?
venous: acid-base assessment, electrolytes, hydration and perfusion
arterial: neonates, respiratory dz, cardiac dz, compensation assessment

where can venous blood draws be performed in large animals?
horses: jugular vein mostly
small ruminants, camelids, cattle: jugular vein (sometimes tail vein in cattle)
pigs: ear veins

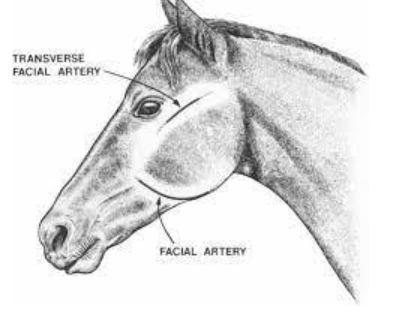
where can arterial blood draws be performed in large animals?
horse: transverse facial, or facial artery (if under GA)
foals: dorsal metatarsal artery
cattle: auricular artery
calves: brachial artery
what is the range for a normal pH?
7.35-7.45
what is the normal range for bicarbonate?
23-27 mEq/L
what are normal PaO2 and PvO2 values?
PaO2: 80-100 mmHg
PvO2: 30-40 mmHg
what are normal PaCO2 and PvCO2 values?
PaCO2: 35-45 mmHg
PvCO2: 40-50 mmHg
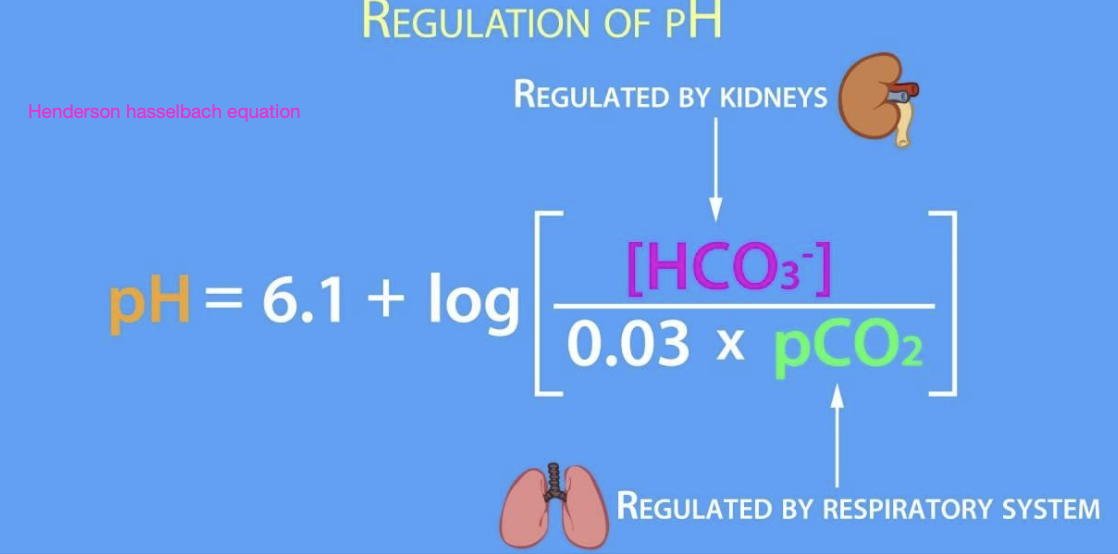
what are the 2 major drivers of pH according to the henderson-hasselbach eqn? what regulates them?
1. bicarbonate (regulated by the kidneys)
2. carbon dioxide (regulated by the respiratory system)
what are other methods for analyzing the pH and the cause of its changes?
anion gap
strong ion difference
fencl equations
what is a buffer?
a compound that can accept a hydrogen ion or donate one enabling minimization of pH changes
what is acidosis?
the pathophysiologic process that leads to increased acid accumulation in the body or decreased base
what is acidemia?
refers specifically to pH being lower than normal due to increased hydrogen ion concentration
what is alkalosis?
pathophysiologic process that leads to decreased acid accumulation or increased base/buffer accumulation
what is alkalemia?
refers specifically to pH being higher than normal due to decreased hydrogen ion concentration
what is a primary acid-base disturbance?
the dominant disturbance that is responsible for the observed pH change
what is a compensatory acid base response?
the body's attempt to correct for the primary disturbance
involves the opposite component that was found to be responsible for dictating pH change
what are the steps to interpreting the pH of a blood gas?
1. assess sample type and quality
2. evaluate the pH
3. determine the nature of the disturbance
how are the causes of acid-base disturbances determined/assessed thru blood gas?
respiratory: pCO2
metabolic: HCO3
how is compensation assessed in acid-base disorders?
HCO3 and pCO2 move in the same direction with simple disturbances
will compensation normalize the pH? does compensation ever overcorrect?
compensation will not normalize pH
compensation will not overcorrect
what blood sample is used to assess compensation in acid-base disorders more precisely?
arterial blood
is the respiratory response or renal response quicker to aid via compensation in acid-base disorders?
respiratory responds faster
what is the acid-base disturbance? expected compensation?
pH: decreased
pCO2: increased
HCO3: increased
respiratory acidosis
expected compensation: increased HCO3
what is the acid-base disturbance? expected compensation?
pH: increased
pCO2: decreased
HCO3: decreased
respiratory alkalosis
expected compensation: decreased HCO3
what is the acid-base disturbance? expected compensation?
pH: decreased
pCO2: decreased
HCO3: decreased
metabolic acidosis
expected compensation: decreased pCO2
what is the acid-base disturbance? expected compensation?
pH: increased
pCO2: increased
HCO3: increased
metabolic alkalosis
expected compensation: increased pCO2
what is respiratory acidosis?
pH <7.35 and PaCO2 >45mmHg
appropriate response: metabolic compensation (renal) to increase/retain HCO3
what are the major causes of respiratory acidosis?
-upper respiratory tract obstruction
-hypoventilation (CNS dz, illness)
-diaphragmatic or thoracic dysfunction
-severe pulmonary disease
what is metabolic acidosis?
pH <7.35 and HCO3 <23mEq/L
appropriate response: respiratory system decreasing pCO2
what are causes of metabolic acidosis?
cause= HCO3 loss
-loss from GI tract (diarrhea)
-high lactate due to poor perfusion
-grain overload
-renal failure
what is metabolic alkalosis?
pH >7.45 and HCO3 >27mEq/L
appropriate response: respiratory system increasing pCO2
what are the causes of metabolic alkalosis?
cause= chloride loss
-GI sequestration
-sweating (horses)
-furosemide
what is respiratory alkalosis?
pH > 7.45 and PaCO2 <35mmHg
appropriate response: metabolic compensation (renal) to decrease HCO3
what are causes of respiratory alkalosis?
hyperventilation due to stress, other factors
fever
CNS disease
what is the anion gap?
the estimate of the amount of 'unmeasured' anions (lactate, ketones, phosphates, sulfates)
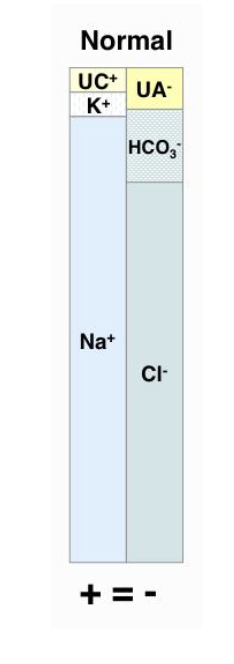
how is anion gap calculated?
AG=(Na+K) - (Cl+HCO3)
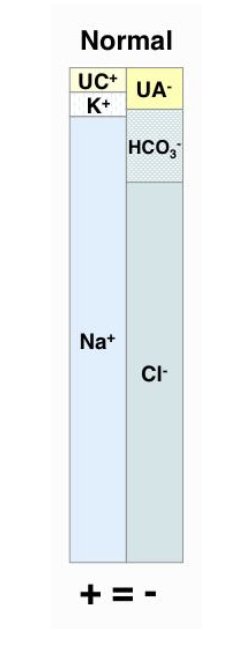
why do we evaluate anion gap?
enables differentiation between the 2 causes of metabolic acidosis (titration acidosis and bicarb loss acidosis)
albumin must be normal to utilize anion gap to differentiate
how does the anion gap differ between titrational acidosis and bicarb-loss acidosis?
titrational: increased anion gap
bicarb loss acidosis: normal anion gap
what is a mixed acid-base disorder?
2 or more disorders occurring at the same time
how are mixed acid-base disorders identified?
-compensatory response (overshoot vs minimal)
-pH is normal but pCO2 and HCO3 are abnormal
-pCO2 and HCO3 changing in opposite directions
-pH change not compatible with primary disorder
what is the cause of an increased PCV and TS?
dehydration
what is the cause of an increased PCV but normal TS?
splenic contraction
what is the cause of an increased PCV and decreased TS?
shock or GI compromise
what is the cause of a decreased PCV and normal/increased TS?
chronic infection or hemolysis

what is the cause of a decreased PCV and TP?
whole blood loss or overhydration
what causes an elevated blood lactate?
decreased perfusion
hypoxemia
dehydration
exercise
t or f: electrolytes are tightly regulated in the body
true
general osmolality of blood is 270-300 mOsm/L
what treatment should be considered for metabolic acidosis?
fluid therapy
if pH <7.2 and/or HCO3 <15mEq/L, consider bicarb administration
what treatment should be considered for respiratory acidosis?
find the cause
improve ventilation
do not give bicarbonate (cannot blow off excess CO2)
what treatment should be considered for metabolic alkalosis?
find and treat the cause
give chloride containing fluids
what treatment should be considered for respiratory alkalosis?
resolves once the cause of hyperventilation is adressed
how is a low bicarbonate deficit corrected?
bicarb deficit= BW (kg) x (normal-actual value) x (0.3 or 0.6)
volume of distribution: 0.3 for adults, 0.6 for young/nursing animals
can give 1/2 of bicarb deficit in first 2 hours, then give remainder over 12 hours
how can bicarbonate be administered?
orally or intravenously
if giving IV, avoid calcium-spiked fluids (will precipitate in line)
can safely used 0.9% saline or 5% dextrose
