FUNCHEM 19 Molecularities of Reactions Mechanisms and Arrhenius Equation
1/7
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
8 Terms
What is a reaction mechanism?
Reaction Mechanism is a sequence of elementary steps that leads to product formation
What is an elementary step?
Elementary step is each individual step in the overall reaction.
What is Molecularity?
Molecularity tells you the number of molecules reacting in each individual step
What is the one Arrhenius Equation

What is another Arrhenius Equation
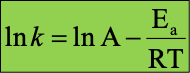
What does the letters mean in Arrhenius equation?
k= rate constant
A= frequency aactor
e= exponential
Ea= exponential activation energy
R= gas constant
T= Temperature in Kelvin
What is the graphical form of the Arrhenius Equation?
y=mx+c
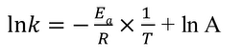
How do you find the activation energy using the Arrhenius equation in the graph
Plot the natural logarithm of the rate constant (ln(k)) on the y-axis against the reciprocal of temperature (1/T) on the x-axis
The result is a straight line where the slope (m) is equal to
-Ea/R, where (R) is the ideal gas constant
Calculate the slope of this line and then use the formula
(Ea = -m x R) to determine the activation energy