Exam Two
1/154
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
155 Terms
endochondral ossification
during embryogenesis, long bones develop from a cartilage mold, by a process including chondrocytes proliferation, hypertrophy, and undergoing apoptosis). The region of apoptosis, the matrix, becomes mineralized.
intramembranous ossification
responsible for the development of flat bones and do not develop from a
What are the three types of bone cells?
osteoblasts, osteocytes, and osteoclasts
osteoblasts
located on the surface of the matrix. Synthesize, transport, and assemble bone matrix and regulate its mineralization
Osteocytes
located within the bone. they are interconnected by a network of cytoplasmic processes through tunnels known as canaliculi. they help to control calcium and phosphate levels, detect mechanical forces, and translate them into biologic activity (mechanotransduction)
osteoclasts
located on the surface of bone. they are specialized multinucleated macrophages, derived from circulating monocytes, which are responsible for bone resorption.
what are the steps for bone remodeling theory?
1) Activation- signals for osteoclasts due to osteocyte apoptosis and/or microdamage formation
2) resorption- removal of bone by osteoclasts
3) Reversal- osteoclasts cease resorption; osteoblasts begin to arrive onsite
4) Formation- production of new matrix by osteoblasts
5) Quiescence- matrix formation stops, and bone surface becomes covered by lining cells
molecules involved in bone multicellular unit
RANK, RANKL, and Osteopotegrin (OPG)
RANK
expressed on osteoclasts precursors. signaling activates osteoclast survival and promotes bone resorption
RANKL
ligand which is expressed on osteoblasts and marrow stromal cells
Osteopotegrin (OPG)
secreted by osteoblasts and can inhibit RANK and RANKL interaction. prevents bone resorption
Achondroplasia
autosomal dominant defect which is caused by a mutation in the FGF receptor 3 which causes the receptor to be in a constant state of activation (retardation of growth plate formation). Causes disproportionate dwarfism with normal trunk length and short extremities, varus and valgus deformities of legs, short fingers and toes, and large head with prominent forehead. Microscopic presentation is narrow and disorganized zones of proliferation and hypertrophy.
Type I Osteogenesis Imperfecta
increased number of fractures; blue sclerae because of this layer of collagen, causing choroid to be visible. caused by a mutation in alpha 1 and alpha 2 chains of type I collagen
Type II Osteogenesis Imperfecta
affected infants are stillborn or die within a few days after birth, in a sense being crushed to death. They are markedly short in stature, with sever limb deformities. Almost all bones sustain a fracture during delivery. caused by a mutation involved in gene for pro-a1 collagen chain in autosomal recessive form of the disease or produce unstable triple helix in autosomal dominant form of disease. manifestation is death in utero or early perinatal period
Osteoporosis
due to decrease in bone mass with a subsequent increase in the risk of fractures. It can be localized to one or few bones (because of disuse) or generalized (involving a majority of the skeletal system). Epidemiology: incidence of osteoporosis increases with age.
Causes of secondary osteoporosis
increased levels of PTH(increase in osteoclast and decrease of estrogen) due to an adenoma, or hyperplasia of the parathyroid glands; diabetes mellitus; Addison disease, malnutrition, or steroid use
pathogenesis of primary osteoporosis
decreased ability of cells to make bones, which occurs with senility of the bone. decreased physical activity. decreased estrogen, which results in increased level of interleukin increase of the level of RANK and RANKL, decrease in OPG. clinical presentation includes vertebral compression fractures with acute back pain and kyphosis; hip fracture
Fracture
broken bone
Complete fracture
extends through bone causing total separation at the site of fracture
compound fracture
bone communicates with skin surface
comminuted fracture
bone broken into many smaller fragments at the site of fracture
displaced fracture
edges of bone at fracture site are no longer aligned
pathologic fracture
fracture occurring at the site of another form of pathology (ex: site of a tumor metastasis)
spiral fracture
caused when torque is directed along the axis of the shaft of a bone
Healing of fractures
following a fracture, ruptured blood vessels results in a hematoma, which fills fracture gap. The clotted blood provides a fibrin mesh, sealing off the fracture site and creating a scaffold for the influx of inflammatory cells and fibroblasts and new capillaries. Simultaneously, degranulated platelets and migrating inflammatory cells release PDGF, TGF-B, FGF, and other factors to stimulate osteoclastic and oteoblastic activity. After a week, a mass of predominantly uncalcified tissue- soft callus- provides anchorage between ends of fractured bone. after 2 weeks, soft callus is transformed into a bony callus. the activated osteoprogenitor cells deposit woven bone
Pathological Fracture
a fracture die to another underlying disease of the bone. Metastatic and primary tumors are not the only cause of pathologic fractures.
Arthritis
inflammation of the joint
osteoarthritis
referred to as degenerative joint disease, occurs as a result of degeneration of the articular cartilage, with gradual onset of symptoms after 40 years of age
pathogenesis of primary osteoarthritis
normal articular cartilage undergoes turnover; however, in osteoarthritis, this turnover does not occur. It is due to the wear and tear and other factors, including genetic factors. It can also be caused due to trauma.
What does the upper respiratory system include?
nose, pharynx, and larynx
What does the lower respiratory system include?
trachea, bronchial tree, and lungs
Obstructive lung disease
a disease of the lungs that impairs the ability of air to leave the alveoli during expiration, trapping it. Several diseases, including chronic asthma, emphysema, chronic bronchitis and cystic fibrosis, are grouped because they all entail obstruction to airflow in the lungs
Chronic Obstructive pulmonary disease (COPD)
includes chronic bronchitis and emphysema, in which forced expiratory volume, measured by spirometry, is decreased.
Chronic Bronchitis
very common in smokers. productive cough without a discernable cause for at least 3 months in 2 consecutive years. related to cigarette smoking. toxins in the smoke irritate the airway, resulting in increased production of mucus, which, in turn stimulates hyperplasia of mucous-secreting glands. The obstruction of the airway by mucus, leading to bronchiectasis (vulnerable to infection) or atelectasis (collapse). Pulmonary hypertension.
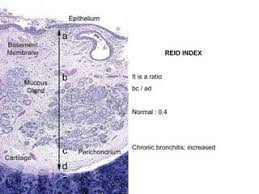
Which disease does this represent?
chronic bronchitis
Emphysema
chronic lung disease in which airspaces distal to terminal bronchioles are enlarged owing to destruction of their walls. The major cause is cigarette smoking. Moderate-to-severe is rare in non-smokers. Smoking causes increased numbers of neutrophils causing increased elastolytic activity leads to destruction of elastic tissue in the walls of distal airspaces, impairing elastic recoil.
signs and symptoms of emphysema
dyspnea, hypoxemia, hyercapnia, hyperventilation. decreased breath sounds and increased expiratory phase on auscultation. Chronic respiratory acidosis with compensatory alkalosis in stable patients. Weight loss may be prominent. Flattened diaphragm and expanded hyperlucent lung field will show up on chest radiograph
Asthma
a disease process characterized by episodic reversible bronchoconstriction of hyperreactive airways in response to various exogenous and endogenous stimuli and is associated with chronic inflammation. it is characterized by hyperreactive airways that constrict in response to stimuli, causing increased airway resistance. In atopic and occupational, the disease process is a type I hypersensitivity reaction involving CD4+ Th2 cells, which release IL-4 and IL-5 stimulating eosinophils and production of IgE.
4 histological findings in asthma
inflammation
bronchial (lumina) narrowing
increased mucus (plugging)
smooth muscle hyperplasia
Immediate response asthma
allergen exposure
allergen binding to IgE on mast cells
mast cell degranulation
edema, mucus hypersecretion, and bronchoconstriction
Delayed Response Asthma
decreased ciliary function and epithelial damage
afferent nerve discharge
efferent (vagal) nerve discharge
bronchoconstriction
atelectasis
collapse of the pulmonary parenchyma. Because of this, airways and alveoli are unable to fill, and blood is shunted from the arteries to the veins without adequate to the veins without adequate oxygenation.
compressive atelectasis
a condition or lesion external to the lungs. compresses the lung and impairs filling of the alveoli upon respiration
Pleural effusion
refers to the accumulation of excess fluid in the pleural cavity. Can be caused by hydrothorax and pyothorax. causes dyspnea (sharp chest pain due to involvement of the parietal pleura that is worsened by coughing or breathing) and dull chest pain due to the involvement of the visceral pleura; or dry cough due to irritation of the pleural surfaces
Tension pneumothorax
defect in the pleura acts as a one-way valve. Air enters the pleural cavity with inspiration but cannot leave it. A needle thoracotomy is required to relieve the tension. May be fatal without treatment
Nontension pneumothorax
air trapped in the pleural cavity
Mesothelioma
malignant tumor of the pleural cavity derived from mesothelial cells. almost always due to the exposure to asbestos. patients first present with pleural effusion or a pleural mass, chest pain, and nonspecific symptoms such as weight loss.
Pneumonia
can be broadly defined as any infection in the lung. Normally, the lung parenchyma remains sterile because of highly effective immune and nonimmune defense mechanisms that extend throughout the respiratory system from the nasopharynx to the alveolar air spaces.
Community acquired typical pneumonia
infection of the lung caused by a bacterial organism that was acquired outside the hospital setting and often follows a viral upper respiratory tract infection.
Bronchopneumonia
patchy distribution of neutrophilic infiltrate and bacterial organisms in one or many lobes
Lobar pneumonia
pneumonia confined to one lobe of the lung
Community acquired atypical pneumonia
pulmonary infection usually due to nonbacterial organism that was aquired outside the hospital setting. patients have only moderate sputum production, lack of alveolar exudates, and only moderate increase in white blood cell count. caused by viruses and can cause bacterial superinfection. signs and symptoms include insidious onset of low-grade fever, nonproductive cough, headache, and myalgias.
Nosocomial Pneumonia
pulmonary infection acquired while hospitalized; usually bacterial, but sometimes hospitalized individuals with severe underlying disease, those who are immunosuppressed, or those on prolonged antibiotic regimens. can be difficult to treat because organisms are often multi-drug resistant to antibiotics
chronic pneumonia
pneumonia of long duration. can be cause by TB, which patients have a granuloma (immune cells organized in a spherical structure surrounded by collagen and fibrinogen) at the periphery of the lung, enlarged hilar lymph nodes. Lesions usually heal on their own and the granulomas become calcified. signs and symptoms include persistent productive cough, fever, chills, loss of appetite, night sweats, and weight loss.
ARDS
a clinical syndrome of progressive respiratory insufficiency caused by diffuse alveolar damage in the setting of sepsis, severe trauma, or diffuse pulmonary infection. Neutrophils and their products have a crucial roles of ARDS by causing endothelial and epithelial injury. characteristics include alveolar edema, epithelial necrosis, accumulation of neutrophils, and presence of hyaline membranes lining the alveolar wall and ducts.
pulmonary embolism
almost all large pulmonary artery emboli are thrombic in origin, usually arising from the deep veins of the lower legs. Risk factors include prolonged bed rest, knee or hip surgery, sever trauma, congestive heart failure, use of oral contraceptives, disseminated cancer and genetic causes of hypercoagulability. most embolisms are silent. symptoms include shortness of breath, chest pain, and cough (may see blood in sputum).
What are the functions of the liver?
protein synthesis including serum proteins that regulate osmotic pressure(albumin, alpha, and beta globulins), lipoproteins, and blood clotting agents
vitamin pathways: these include vitamin A which is taken up, stored, and released by the liver bound to RBP
iron storage and metabolism
processing of nutrients
endocrine function: parts of vitamin D, thyroxin, and growth hormone pathways occur in liver; primary site of insulin and glucagon activity
exocrine function: synthesis and release of bile(digestive fluid)
Structure of the liver
the largest gland and largest internal organ. externally, it is divided into 4 anatomical lobes that are of no physiological significance. Internally, it is organized into functional lobules that have no external expression. The porta hepatis is the hilum of the organ and serves as the entry of the hepatic arteries and portal vein and the exit of hepatic ducts. It is covered by a thin connective tissue capsule.
The liver acinus model
is useful in explaining hepatocyte pathology. The acinus is an oval shaped unit whose short axis is defined by central veins. The hepatocytes are arranged into three concentric zones centered along the sort axis
Zone 1 of acinus
closest to the blood supply
Zone 2 of acinus
furthest from the blood supply and closest to the terminal hepatic venule (central vein)
zone 3 of acinus
hepatocytes are the first to show pathological changes resulting from ischemia whereas zone 1 hepatocytes are the first to react to toxins or bile blockage (bile stasis)
Cirrhosis
diffuse scarring of the liver with nodular regeneration of hepatocytes, resulting in severe disruption of hepatic architecture. can be caused by viral hepatitis, hepatitis C, alcohol abuse, nonalcoholic fatty liver disease(NAFLD), or nonalcoholic steatohepatitis(NASH)
Complications of cirrhosis
portal hypertension, ascites, hepatocellular dysfunction, portal vein thrombosis, and hepatocellular carcinoma
portal hypertension
the portal vein normally drains into the liver. if the liver is scarred, intrahepatic vascular tone increases, causing an increased resistance to portal venous pressure. It forces blood returning to the heart to take alternate routes such as esophageal varices, hemorrhoids, and caput medusae. It contributes to the development of ascites and splenomegaly.
Ascites
accumulation of fluid in peritoneal cavity. this occurs as the result of portal hypertension and hypoalbuminemia
Hepatic Failure
occurs in one of three situations: massive hepatic necrosis, chronic liver disease, or widespread but not fatal injury to the hepatocytes. massive hepatic necrosis is often the result of fulminant hepatitis or toxic injury.
complications of hepatic failure
hepatic encephalopathy, hepatorenal syndrome, jaundice, hyperammonemia, coagulopathy, hypoglycemia, and infections.
Hepatic encephalopathy
complex neuropsychiatric syndrome that complicates advanced liver disease
HAV
the most common cause of acute hepatitis, but no risk of cirrhosis. hepatic injury due to immune response to infected hepatocytes, fecal-oral transmission, fever, malaise, and anorexia. acute only; never chronic. lifelong immunity, full recovery. vaccination for long-term protection
HBV
major cause of acute and chronic liver disease. transmission-shared blood, sexual contact. vaccines for lifelong immunity. clinical courses: acute, fulminant is rare, chronic (necrosis and inflammation > 6 months, higher risk of cirrhosis and hepatocellular carcinoma)
HCV
is the single virus that is more often chronic than not(almost never detected acutely; 85% or more if patients develop chronic hepatitis, 20% of whom will develop cirrhosis). it is a common cause of chronic hepatitis and cirrhosis
treatment of hcv
interferon-alpha and ribavirin
Hepatocellular Carcinoma
arises from hepatocytes and a consequence of HBV infection. HBV viral genome integrates into hepatocyte DNA., HBV X protein inactivates tumor suppressor proteins (TP53).
Alcoholic Fatty liver disease
steatosis-fat accumulation in hepatocytes. in hepatocytes, ethanol increases fatty acid and triglyceride synthesis, decreases fatty acid oxidation, and impairs release of lipoproteins. grossly, the liver appears enlarged and yellow. reversible if alcohol use stops
alcoholic hepatitis
pathology: hepatocyte ballooned cells and necrosis, hyaline inclusions(Mallory Bodies), inflammation, and necrosis. symptoms and signs: malaise, anorexia, fever, right upper quadrant pain, and jaundice.
Cholelithiasis
cholesterol gallstones in the gallbladder. there is supersaturation of bile and cholesterol. Cholesterol isn’t dispersed and cholesterol is toxic to the gallbladder, promoting hypomotility, which results in nucleation of cholesterol. Hypersecretion of mucus traps cholesterol crystals, forming stones. risk factors include obesity, female, fertile, older than 40 years of age, and native American ancestry
structure of the pancreas
it is an endocrine and exocrine glandular organ found deep to the transverse colon. Its expanded head rests in the C-loop of the duodenum and its body and tail extend towards the spleen. The pancreatic duct extends the length of the gland and empties into the hepatopancreatic ampulla. from the ampulla, pancreatic exocrine secretions (and bile) are released into the duodenum via the hepatopancreatic sphincter. It has a poorly developed connective tissue capsule. Septa extending from the capsule divide the gland into lobules.
exocrine portion of the liver
is compromised of serous glands that drain to the duodenum via pancreatic ducts. These glands synthesize and secrete digestive enzymes.
endocrine portion of the liver
located in the pancreatic isles and synthesize and secrete the hormones insulin, glucagon, and somatostatin to the systemic circulation.
histologic slide of the pancreas
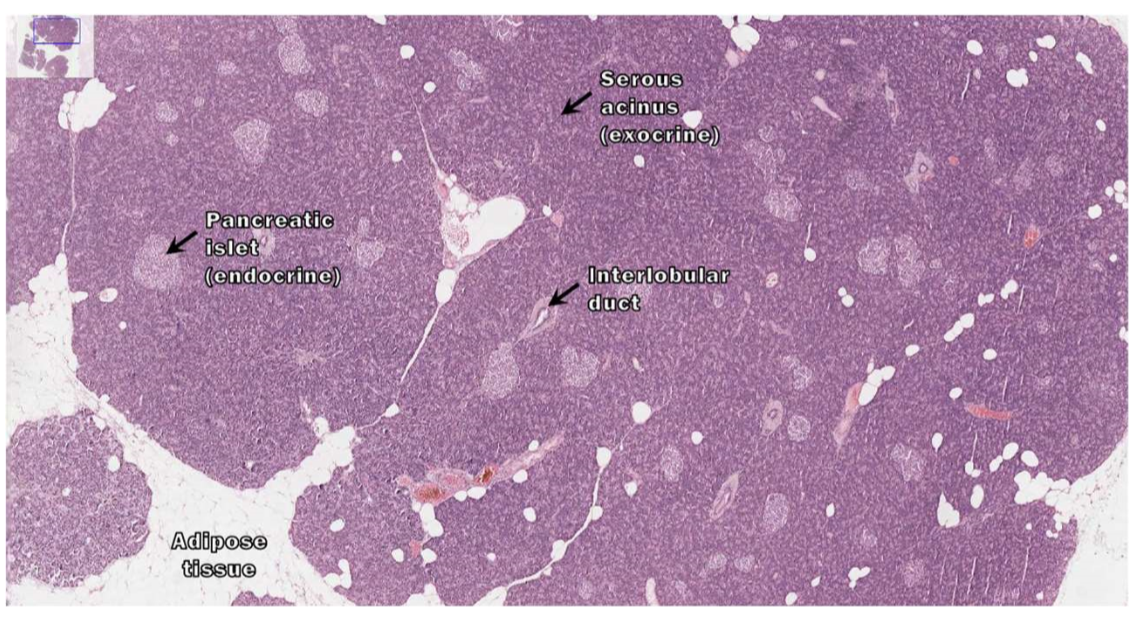
What are the two cells of the exocrine pancreas?
acinar cells and ductal cells
What do acinar cells secrete?
digestive enzymes (mostly inactive proenzymes that are stored in zymogen granules). a-Amylase is a major enzyme secreted a
duct cells
secrete bicarbonate (HCO3-)
acute pancreatitis
characterized by inflammation and reversible parenchymal damages that ranges from focal edema and fat necrosis to widespread parenchymal necrosis and hemorrhage. the clinical presentation varies widely from mild abdominal pain to rapidly fatal vascular collapse which is reversible.
pathogenesis of acute pancreatitis
appears to be caused by autodigestion of the pancreas by inappropriately activated pancreatic enzymes. Activated trypsin is capable of converting other zymogen forms of pancreatic enzymes to their active forms. premature activation of trypsin within the substance of the pancreas can unleash these proenzymes (phospholipases and elastases), leading to tissue injury and inflammation. mainly causes include duct obstruction and acinar cell injury
acute hemorrhagic pancreatitis
signs and symptoms include severe epigastric pain, referred to upper pack, nausea, and vomiting. diagnosis is elevated serum levels of amylase and lipase. treatment includes iv fluids, pain medication, fasting for 1-2 days, and sometimes surgery to remove damaged tissue. it may cause diffuses alveolar damage and disseminated intravascular coagulation
chronic pancreatitis
characterized by irreversible parenchymal damage and scar formation; clinical presentations include chronic malabsorption (due to pancreatic exocrine insufficiency and diabetes mellitus (due to islet cell loss). in many patients, it’s the result of recurrign bouts of acute pancreatitis due to causes such as alcohol abuse and gallstones. progressive destruction of parenchyma, fibrosis, and chronic inflammation. treatment includes iv fluids, pain management, oral pancreatic enzymes, and dietary changes.
controlled cell proliferation
hyperplasia. regulated growth and division of cells in response to physiological demands or tissue repair
neoplastic cell proliferation
autonomous, excessive, and disorganized. uncontrolled growth and division of cells that is characteristic of cancer. cells do not respond to normal regulatory signals, leading to tumor formation and invasion into surrounding tissues.
proto-oncogenes
normal genes which have the potential to become oncogenes. they may be altered by mutations or amplification to become oncogenes.
oncogenes
cancer-causing genes that encode cancer-causing oncoproteins.
tumor suppressor genes
genes or anti-oncogenes, encode tumor suppressor proteins that inhibit cell division. this function is lost in cancer
telomerase
has a role of eventually wearing down and causing eventual cell death.
Stepwise transition from normal tissue to cancer
normal, hyperplasia, dysplasia, and cancer
Explain the progressive transformation from normal to malignant tissue
an epithelial cell becomes initiated: it acquires a mutation in a cancer-related gene. appearance: normal gene
hyperplasia/dysplasia/intraepithelial neoplasia: the initiated cell has proliferated more than neighboring cells. The basement membrane (yellow) is still intact.
the tumor cells have invaded through the basement membrane. this is an invasive, malignant cancer.
Dysplasia
disorderly proliferation. a term used to describe the presence of abnormal cells within a tissue or organ
neoplasia
new growth
what are the fours points in which benign tumor and malignant tumors differ?
growth rate: benign grow slowly and have a well-defined growth pattern, while malignant tumors grow rapidly and have an invasive growth pattern.
spread: benign tumors remain localized and do not invade nearby tissues or spread to distant sites, whereas malignant tumors have the ability to invade surrounding tissues and spread to other parts of the body through a process called metastasis
microscopic and gross appearance: benign tumors resemble the normal tissue fro which they arise and have a uniform appearance under the microscope. malignant tumors have an abnormal appearance with varying degrees of differentiation.
degree of differentiation: Benign tumors are well-differentiated, meaning that the cells closely resemble the normal cells of the tissue they arise from. malignant tumors can range from well-differentiated to poorly differentiated or undifferentiated.
How can cancer cause illnesses and eventually kill?
benign neoplasm: growth that is localized to one place
malignant neoplasm: growth that invades surrounding tissue and, in most cases, can metastasize (spread) to distant organs.