mass number and isotopes
1/8
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
9 Terms
What would we call this atom?
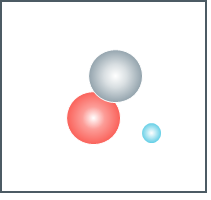
hydrogen-2
mass number
is the number of protons and neutrons
what property of an atom determines which element it is?
proton number
Chemists measure mass at the atomic level using a unit called the…
This unit is exactly equal to…
atomic mass unit
1/12th of carbon-12 atom
what is the mass of an isotope, measured in?
AMU
what is The mass of an isotope called?
relative isotopic mass
The relative abundance of an isotope
is the number of atoms of that isotope that exists on Earth, as a percentage of all the atoms of that element
Relative atomic mass
is the average mass of one atom of an element relative to one twelfth of the mass of a carbon-12 atom
The relative abundances of all the naturally-occurring isotopes of an element must sum to...
100 percent