The Atom
1/21
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
22 Terms
Law of Conservation of Mass
Matter is not created nor destroyed in any ordinary chemical or physical change
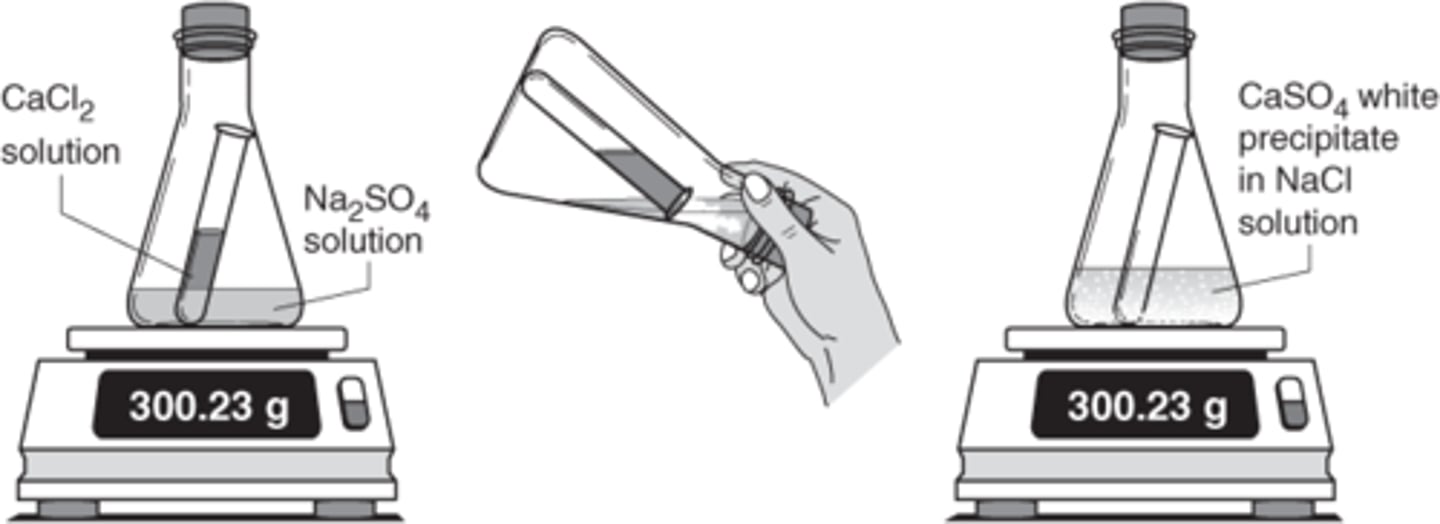
Law of Definite Proportions
A chemical compound contains the exact same proportions by mass no matter the size of the sample of the compound
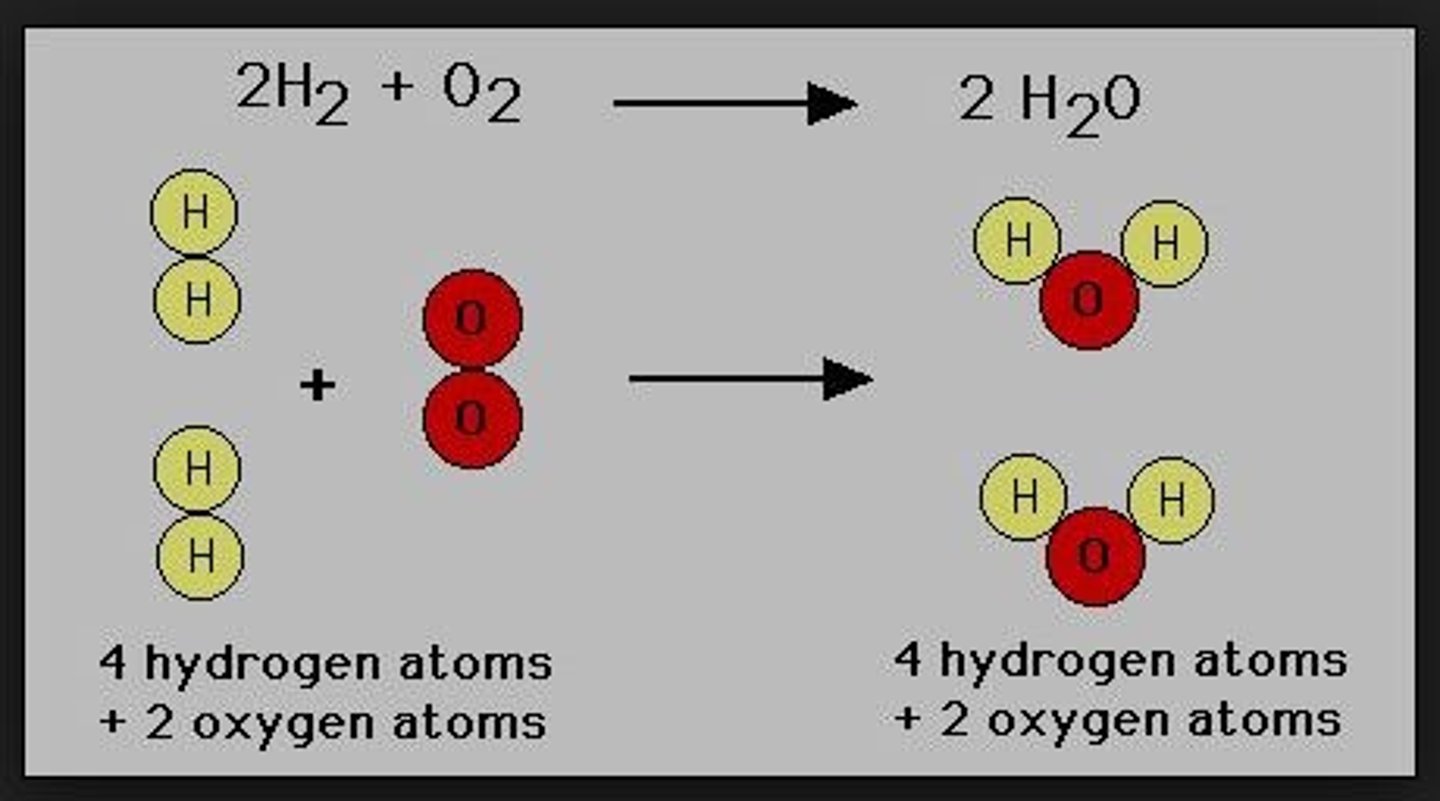
Law of Multiple Proportions
If 2 or more different compounds are composed of the same 2 elements, then the ratio of the masses of the 2nd element combined with a certain mass of the 1st element is always a ratio of small whole numbers
Democritus (400 BC)
Named the atom -- 'atomos', meaning small indivisible particle
Dalton ~1803
Concluded that:
All matter is made of atoms
Atoms of a given element are identical in size, mass and properties
Atoms cannot be divided, created or destroyed
Atoms of different elements combine in simple whole# ratios to form compounds
In chemical reactions atoms are combined, separated or rearranged
J.J. Thomson/Millikan
-performed cathode ray tube experiment
-discovered the electron with a mass of less than 1 and a negative charge
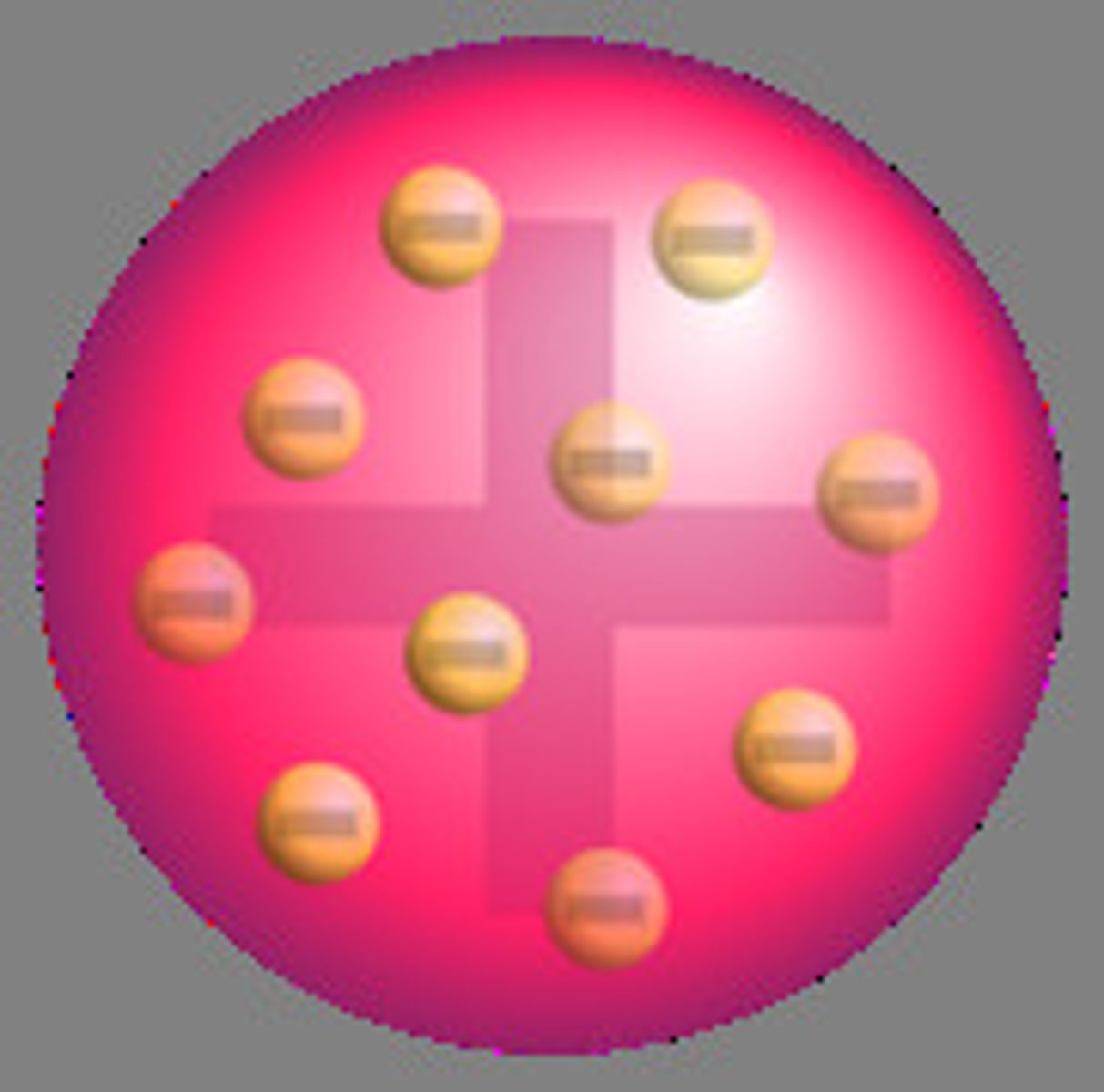
Plum Pudding Model
Concluded that electrons were negatively charged in a positive cloud
E. Rutherford
Fired alpha particles at gold foil
Positive Nucleus Model
Discovered atom was mostly empty space with
positive center
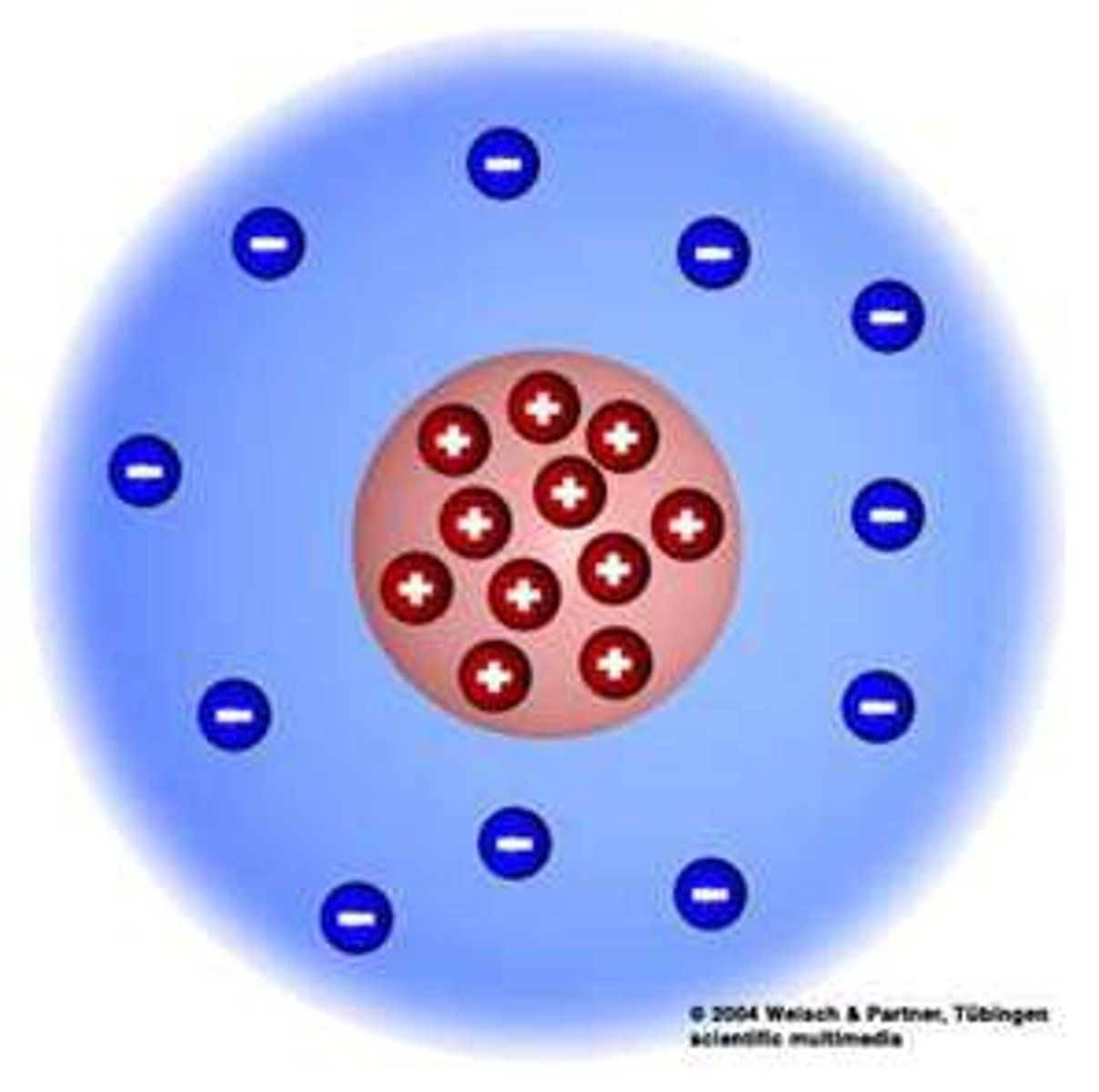
Modern Atomic Theory
The atom is smallest particle of an element that retains the chemical properties of that element, and could be seperated into subatomic particles
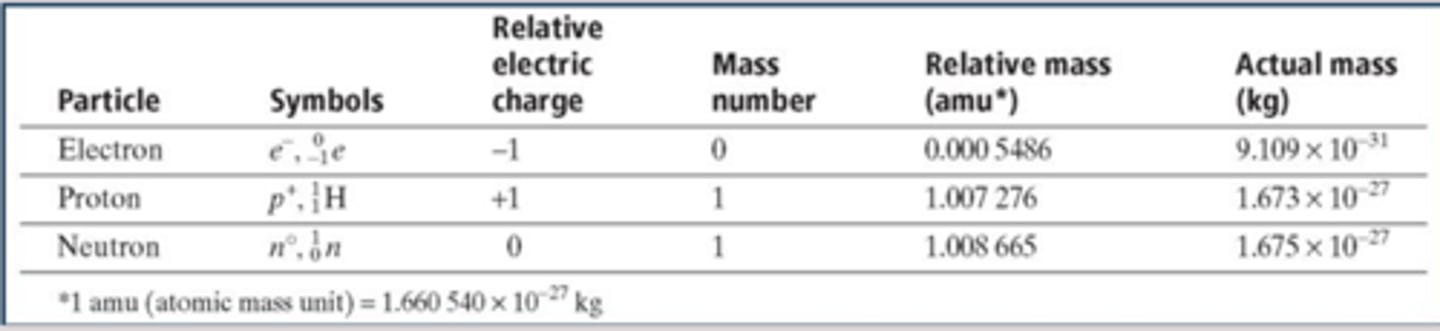
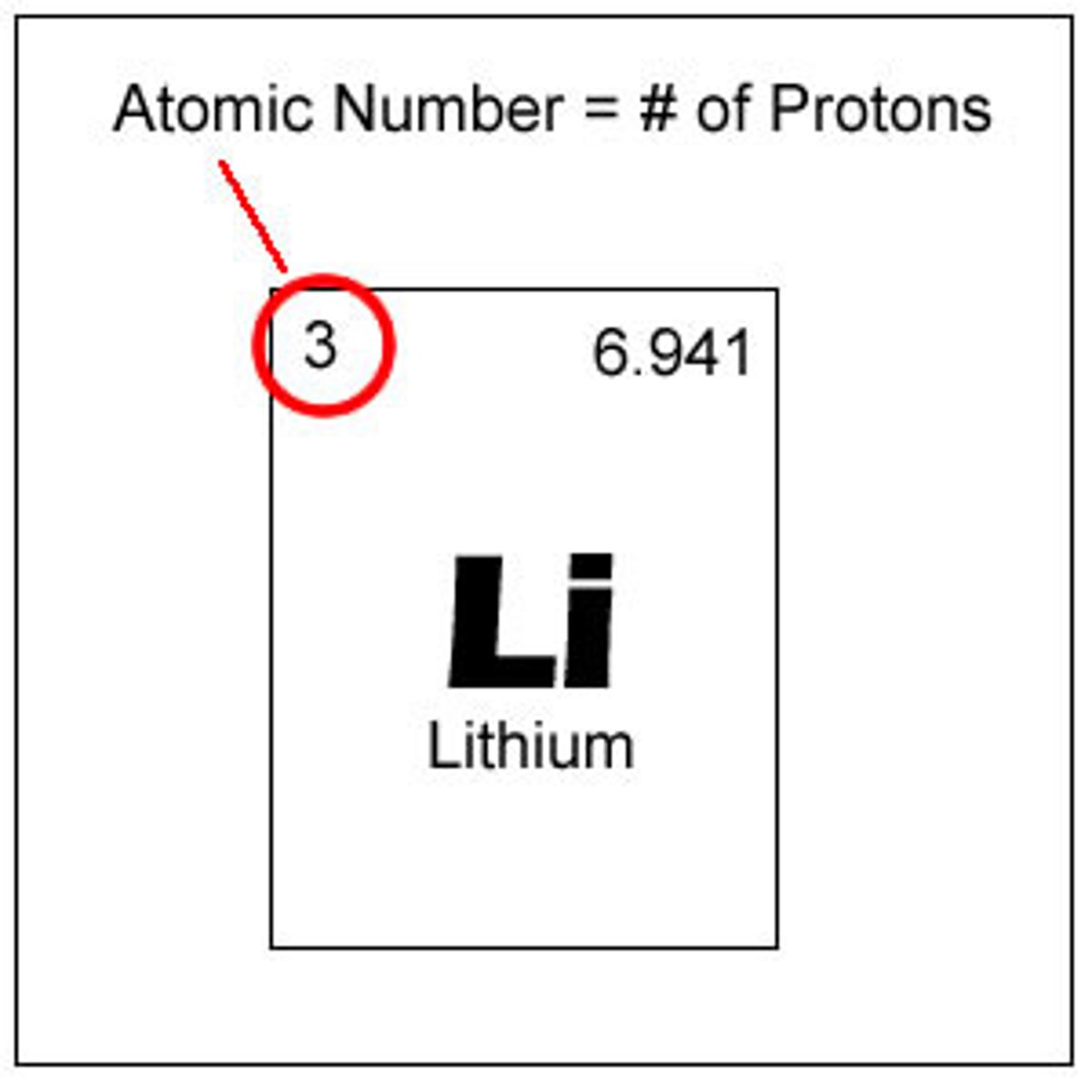
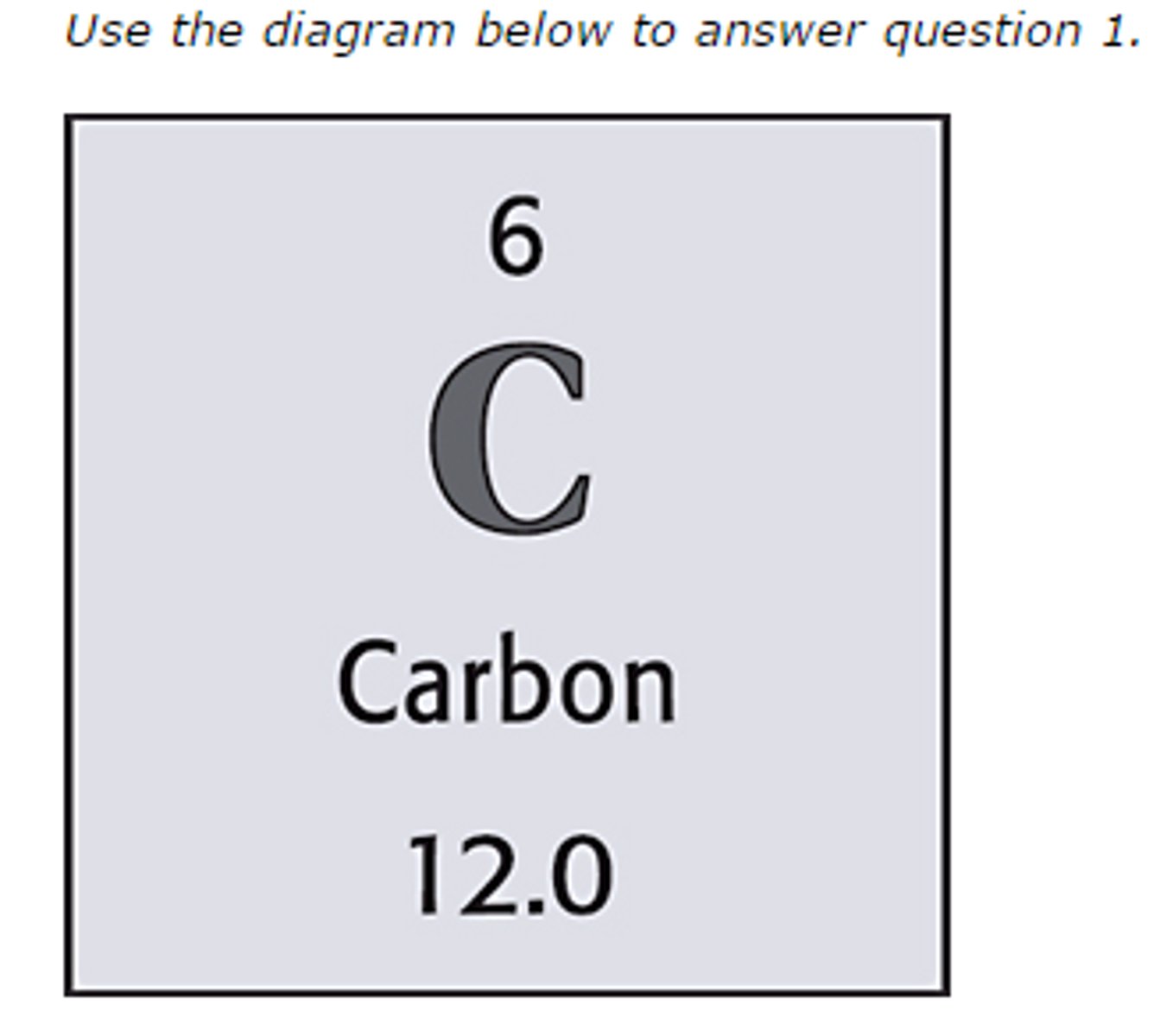
Atomic Number
Symbol; the number of protons in the nucleus of an atom; Identifies each element
Mass Number
the sum of the number of neutrons and protons in an atomic nucleus; Mass# - Atomic# = # of neutrons

12 protons, 12 electrons, 12 neutrons
Find the subatomic particles of Magnesium (Mg)

14 protons, 14 electrons, 14 neutrons
Find the subatomic particles of Silicon (Si)

Isotopes
Atoms of the same element that have different masses; same # of protons and electrons; different # of neutrons
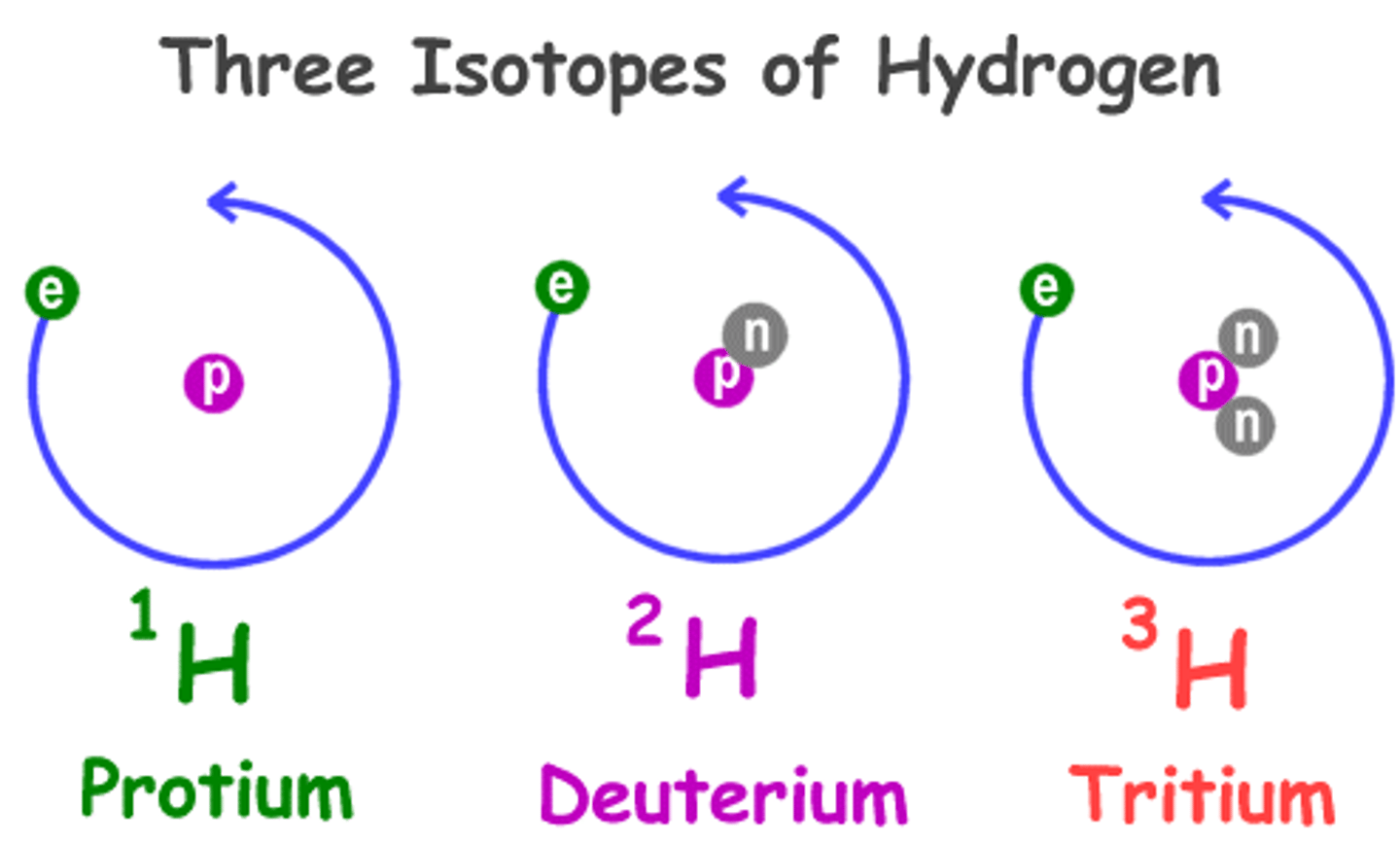
Hyphen Notation
the mass number is written with a hyphen after the name of the element (Ex: Nitrogen-15)

Nuclear Symbol
shows the atomic number, mass number and charge(if any)
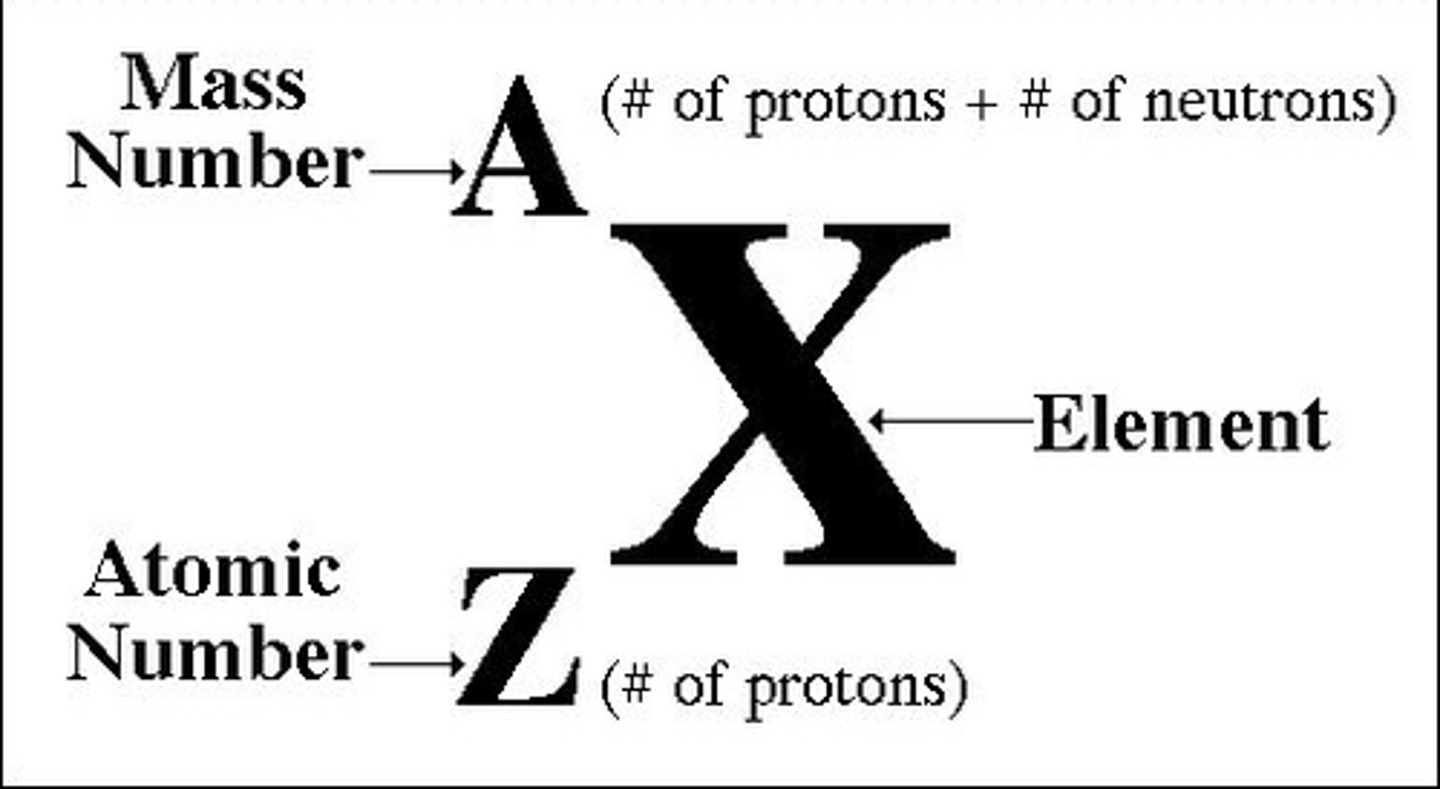
Same protons & electrons; 2 neutron difference
State the subatomic particles of Carbon-14 vs Carbon-12
Same protons and neutrons, different electrons
State the subatomic particles of O^2 vs O
Average Atomic Mass
the weighted average of the masses of all naturally occurring isotopes of an element
Turn % into a decimal
Multiply by Mass
Add together
How to calculate atomic weight
2 electron difference, same neutrons and protons
State the Subatomic Particles of O^-2 vs O (^2 is an exponent)
