Solutions
1/88
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
89 Terms
What is a solution?
homogenous mixture of two or more substances
What is a solvent?
substance that does the dissolving; highest proportion
What is a solute?
Substance being dissolved; minority component(s)
What are common types of solutions?
gaseous solution, liquid solution, solid solution
What is solubility?
when solute dissolves in solvent
Solubility depends on
nature's tendency toward mixing and the types of intermolecular attractive forces
Why do ideal gases mix?
mixing increases their entropy
What is entropy?
measure of energy dispersal in a system
What does energy have the tendency to do with volume?
spread out as large as it is allowed
If solvent-solute interactions are stronger than solvent-solvent and solute-solute interactions, what happens?
a solution forms (not driven by entropy)
If solvent-solute interactions are equal to solvent-solvent and solute-solute interactions, what happens?
solution forms, driven by entropy
If solvent-solute interactions are weaker than solvent-solvent and solute-solute interactions, what happens?
solution may/may not form
What is the first step of energy changes in solution formation?
Separating solute into constituent particles
What is the second step of energy changes in solution formation?
Separating solvent particles from each other to make room for solute particles
What is the third step of energy changes in solution formation?
Mixing solute particles with solvent particles
Separating the solute into its constituent particles is endothermic or exothermic?
endothermic; energy is needed to overcome bonds/forces
Separating the solvent particles from each other to make room for solute particles is endothermic or exothermic?
endothermic; energy is needed to separate/overcome forces attaching them
Mixing the solute particles with solvent particles is endothermic or exothermic?
exothermic; bonds form from mixing, releases more energy than absorbed; excess energy being heat
Exothermic dissolving occurs when?
energy released from making solute-solvent attractions is greater than energy required to break pure solute and solvent attractions
Endothermic dissolving occurs when?
energy released from making solute-solvent attractions are less than energy required to break pure solute and solvent attractions
What is the heat of hydration?
Delta H solvent and delta H mix combined; heat released when one mole of gaseous ions dissolves in water
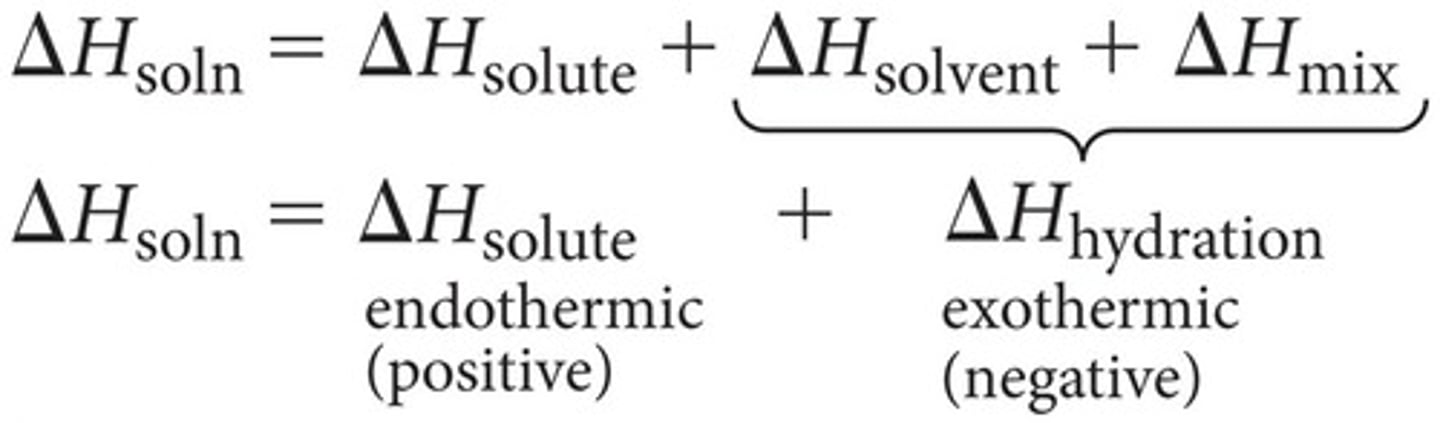
What is lattice energy?
attractive forces between ions; Delta H solute = - delta H lattice energy
When ions dissolve in water, what do they become?
hydrated; surrounded by water molecules
Ion-dipole attractions causes heat of hydration to be
exothermic
Lattice energy is always
exothermic
What is an exothermic solution in terms of ΔH?
ΔH < 0 (-)
What is the relationship between hydration energy and solute separation energy in an exothermic solution?
Hydration energy > solute separation energy
What is an endothermic solution in terms of ΔH?
ΔH > 0 (+)
What is the relationship between hydration energy and solute separation energy in an endothermic solution?
Hydration energy < solute separation energy
When is ∆H solution = 0? (thermoneutral)
hydration energy = solute separation energy
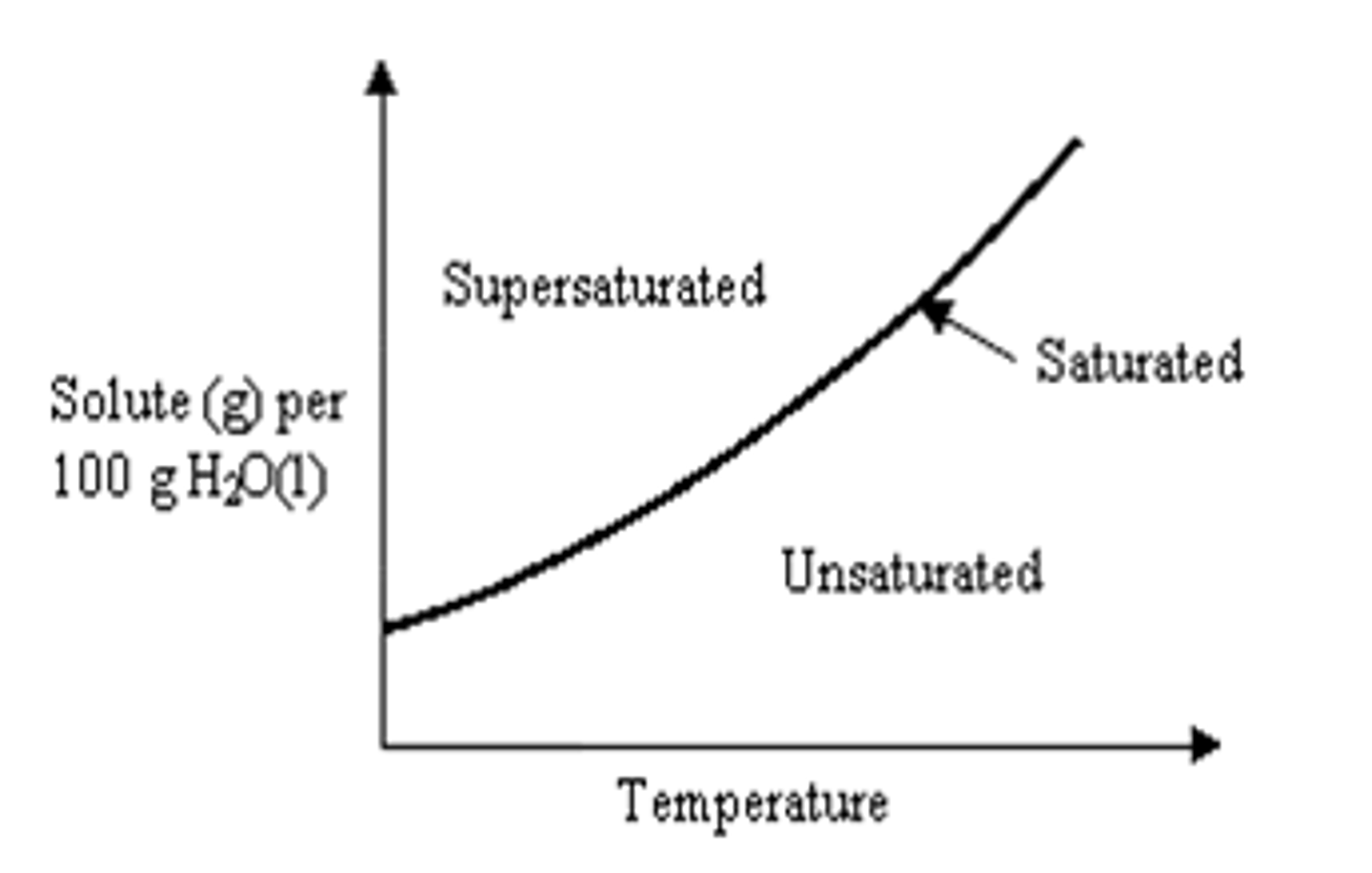
What is a saturated solution?
solute and solvent are equal (dynamic equilibrium)
What happens when you add more solute to a saturated solution?
It will not dissolve; solute and solvent are in equilibrium
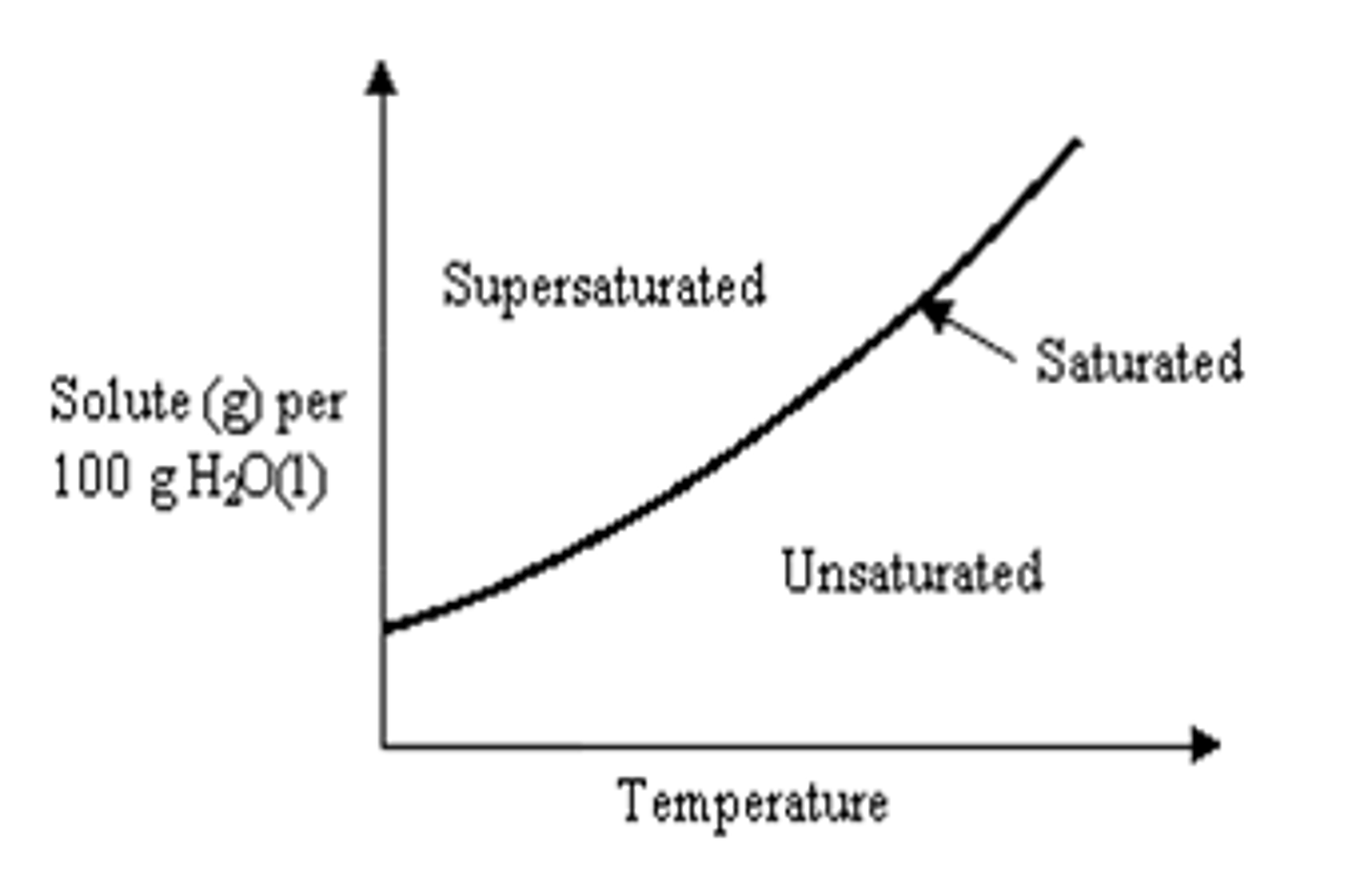
What is an unsaturated solution?
contains less solute than saturation
What happens if you add more solute to an unsaturated solution?
It will dissolve (at given temperature)
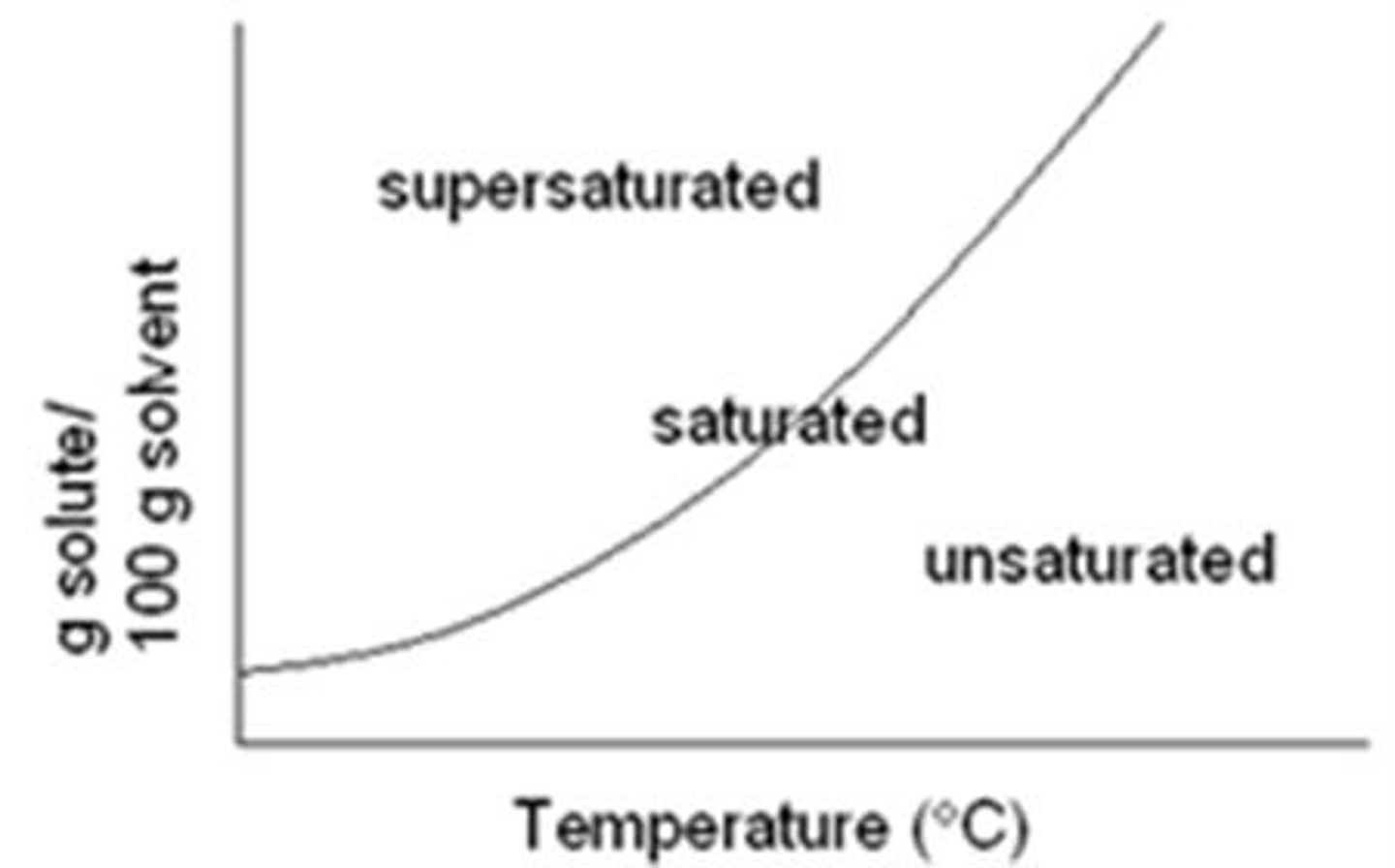
What is a supersaturated solution?
contains more solute than saturation

What happens if you add more solute to a supersaturated solution?
solute crystallizes (precipitates) out; it is already holding more solute than normal

Define solubility
grams of solute that dissolve in 100g of water
How does temperature affect the solubility of solids?
as temperature increases, solubility increases
How does temperature affect the solubility of gases?
as temperature increases, solubility decreases
Saturated Solubility curve
on the line
Unsaturated solubility curve
below the line
Supersaturated solubility curve
above the line
What is recrystallization?
purify solid by dissolving it in hot solvent until it is saturated; forms crystals
How does pressure affect the solubility of gas?
as pressure increases, solubility increases in liquid
How does pressure affect the solubility of a solid?
no effect
What is Henry's Law?
the solubility of a gas in a liquid is directly proportional to the partial pressure of that gas (on the surface of the liquid)
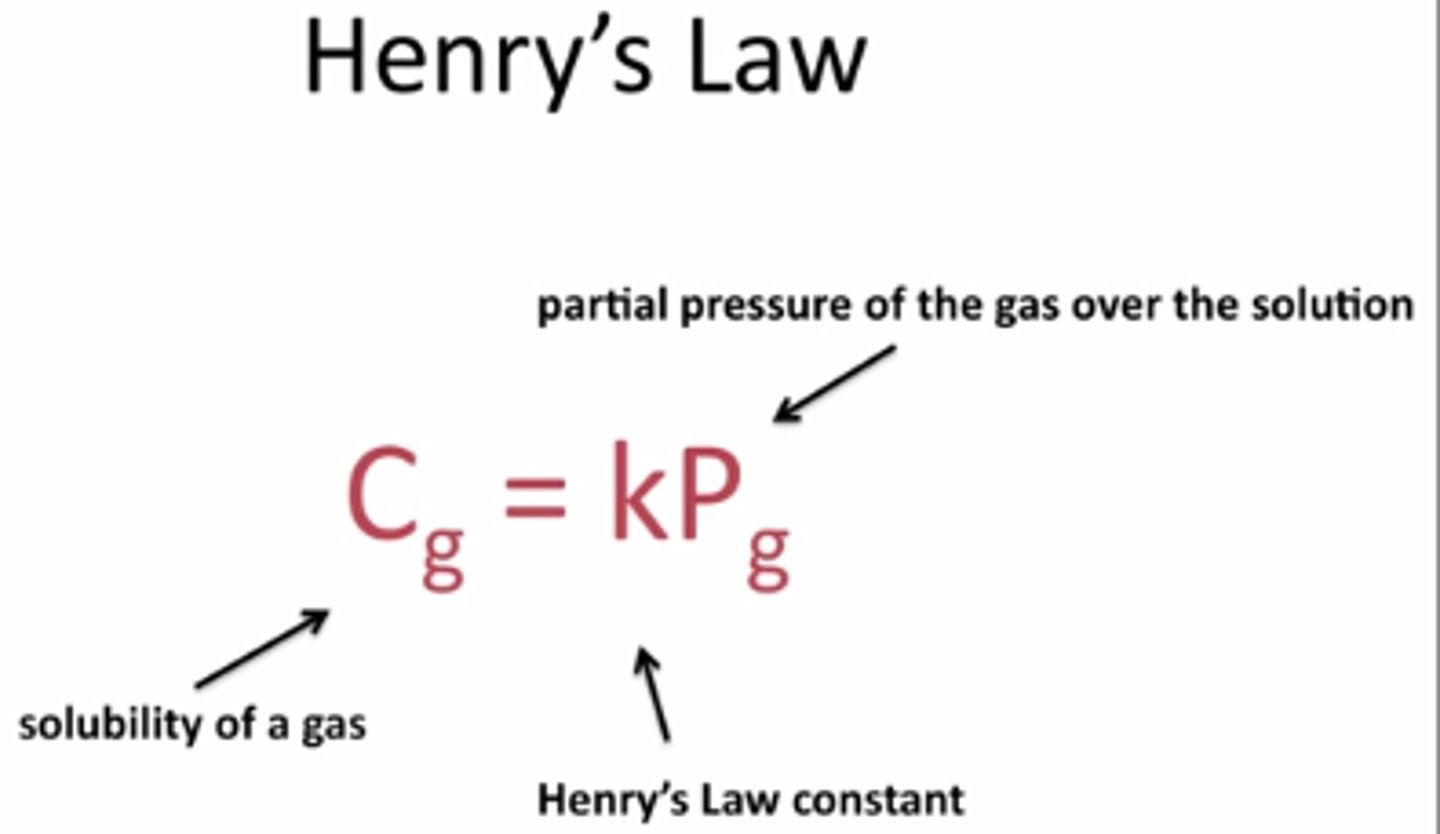
Why does ammonia have a larger Henry's law constant than nonpolar gases?
Ammonia is polar > more soluble > larger constant
What is concentration?
amount of solute in a solution
What is molarity?
amount of solute divided by volume of solution
Molarity depends on
volume
Volume depends on
temperature and pressure
What is molality?
moles of solute/kg of solvent
Molality depends on
mass of solvent
What is parts by mass?
mass solute/mass solution x multiplication factor
What is percent by mass?
mass of solute/mass of solution x 100
What is parts per million (ppm)?
mass of solute/mass of solution x 10^6
What is parts per billion (ppb)?
mass of solute/mass of solution x 10^9
What is parts by volume?
volume solute/volume solution x multiplication factor
What is mole fraction?
moles of solute/total moles of solution
Why is salt put on icy roads?
Salt lowers water's freezing point (so water stays liquid), so ice can melt even below 0 °C
What is a colligative property?
depends on number of solute particles, not the type of particle (COUNT, NOT KIND)
What are the four colligative properties?
vapor pressure lowering, boiling point elevation, freezing point depression, osmotic pressure
What is the vapor pressure of a liquid?
the pressure exerted by the gas when a liquid phase and gas phase are in equilibrium
What is vapor pressure lowering?
When solute is added, fewer solvent molecules escape > solution's vapor pressure is less than pure solvent (MORE SOLUTE, LESS VAPOR); occurs at all temperatures
How does a nonvolatile solute affect vapor pressure?
it lowers vapor pressure, slows down vaporation
How does a concentrated solution affect vapor pressure?
MORE SOLUTE (concentration) LESS VAPOR
What happens when both solute and solvent are volatile?
both evaporate; both add up to total vapor pressure
What equals total vapor pressure?
vapor pressure of solute and vapor pressure of solvent
What are ideal solutions?
solute-solvent = solute-solute + solvent-solvent (follows Raoult's law)
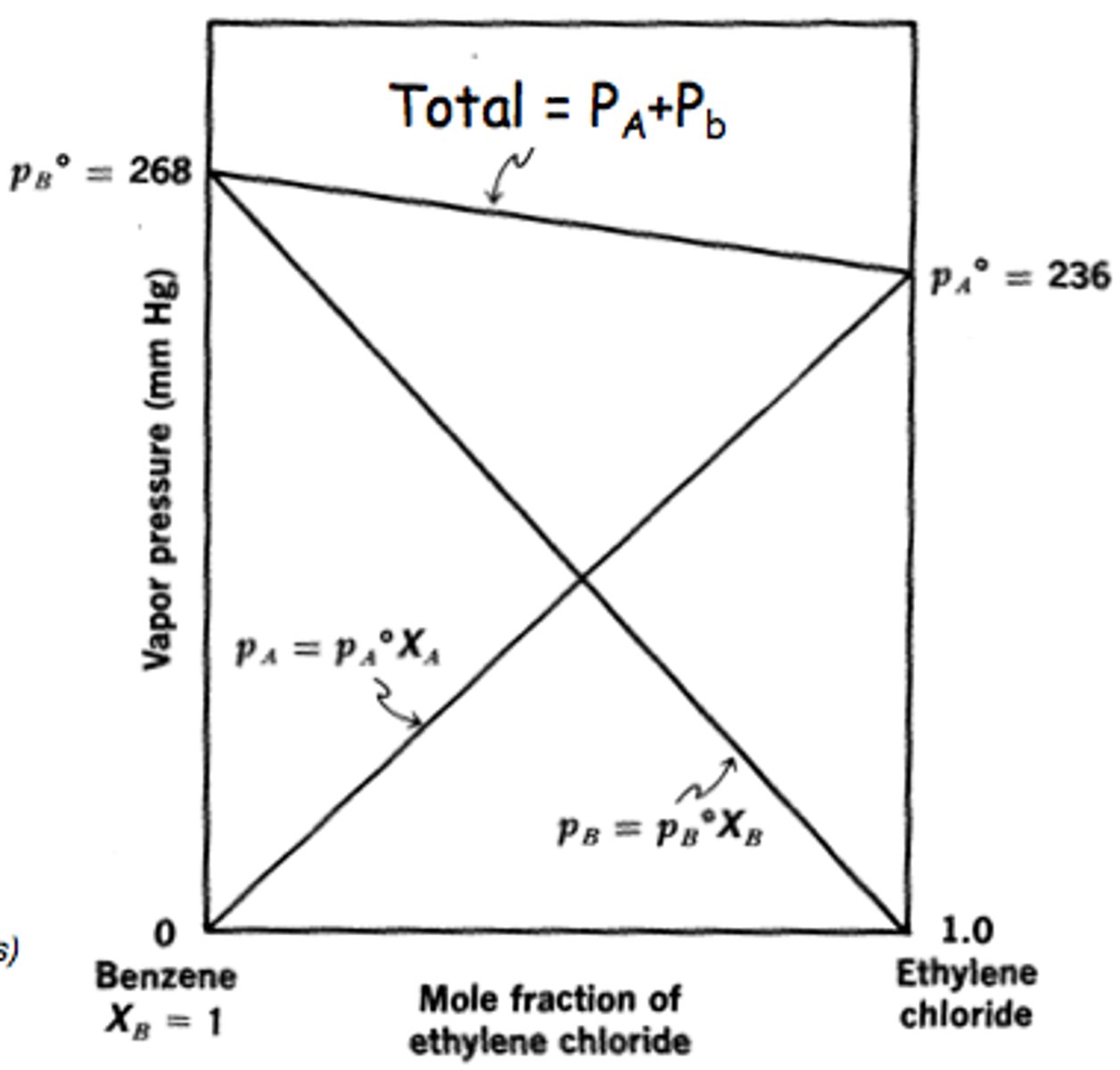
What are nonideal solutions?
solute-solvent > or < solute-solute + solvent-solvent
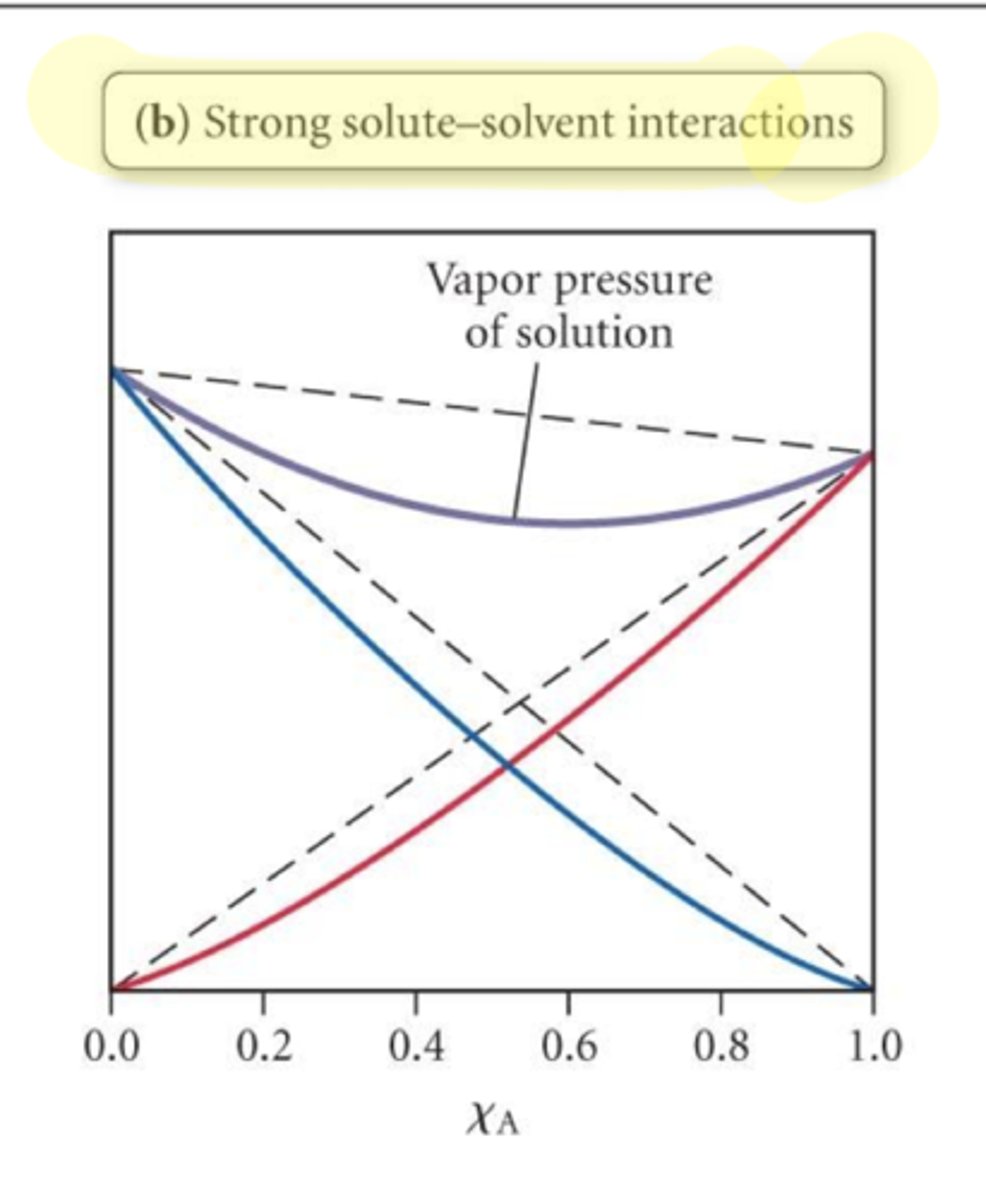
Stronger solute-solvent interactions result in
less total vapor pressure; individual pressures lower than ideal
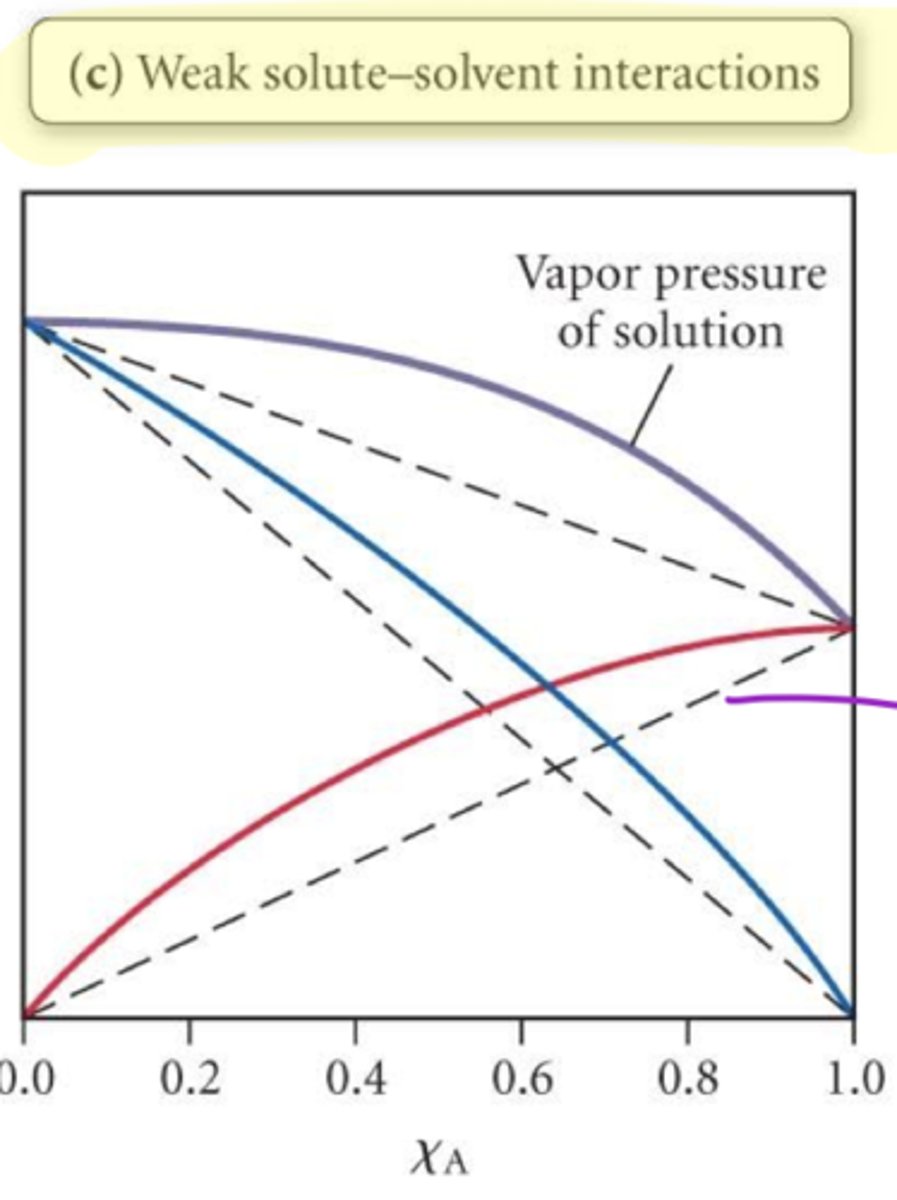
Weaker solute-solvent interactions result in
more total vapor pressure; higher than predicted
How does vapor pressure lowering affect boiling points?
boiling point increases as temperature is required to increase
How does vapor pressure lowering affect freezing points?
freezing point decreases as temperature is required to decrease
What is freezing point depression?
adding a solute to a solvent decreases the freezing point of the solvent; solution will be lower than solvent
What is boiling point elevation?
the temperature difference between a solution's boiling point and a pure solvent's boiling point; solution higher than solvent (nonvolatile)
What is osmosis?
movement of water from high to low concentration
What is osmotic pressure?
external pressure needed to stop osmotic flow
Why are electrolytes treated differently for colligative properties?
Electrolytes dissociate into ions > produce more particles > greater effect on properties than nonelectrolytes
What are colloids?
mixtures that contain tiny particles finely dispersed in a medium and do not separate on standing
Can colloids pass through a semipermeable membrane?
no, particles are too large to pass (but tiny to stay suspended/not settle on ground)
How are hydrophilic (polar) colloids stabilized?
attraction to solvent (like water molecules) to keep them evenly dispersed
How are hydrophobic (nonpolar) colloids stabilized?
charged surface repulsions (due to like charges, prevents clumping)
What are two key behaviors of colloids?
Tyndall effect and Brownian motion
What is the Tyndall effect?
the scattering of visible light by colloidal particles
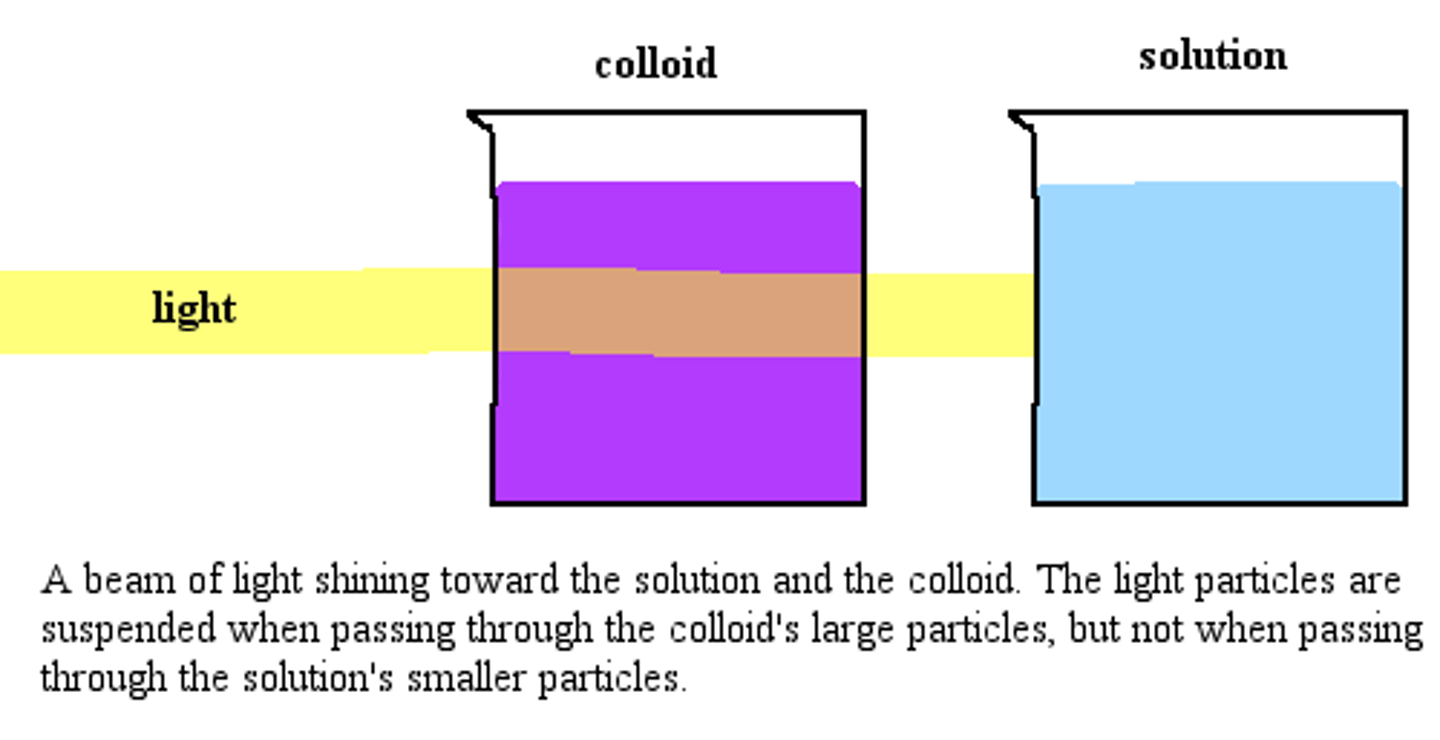
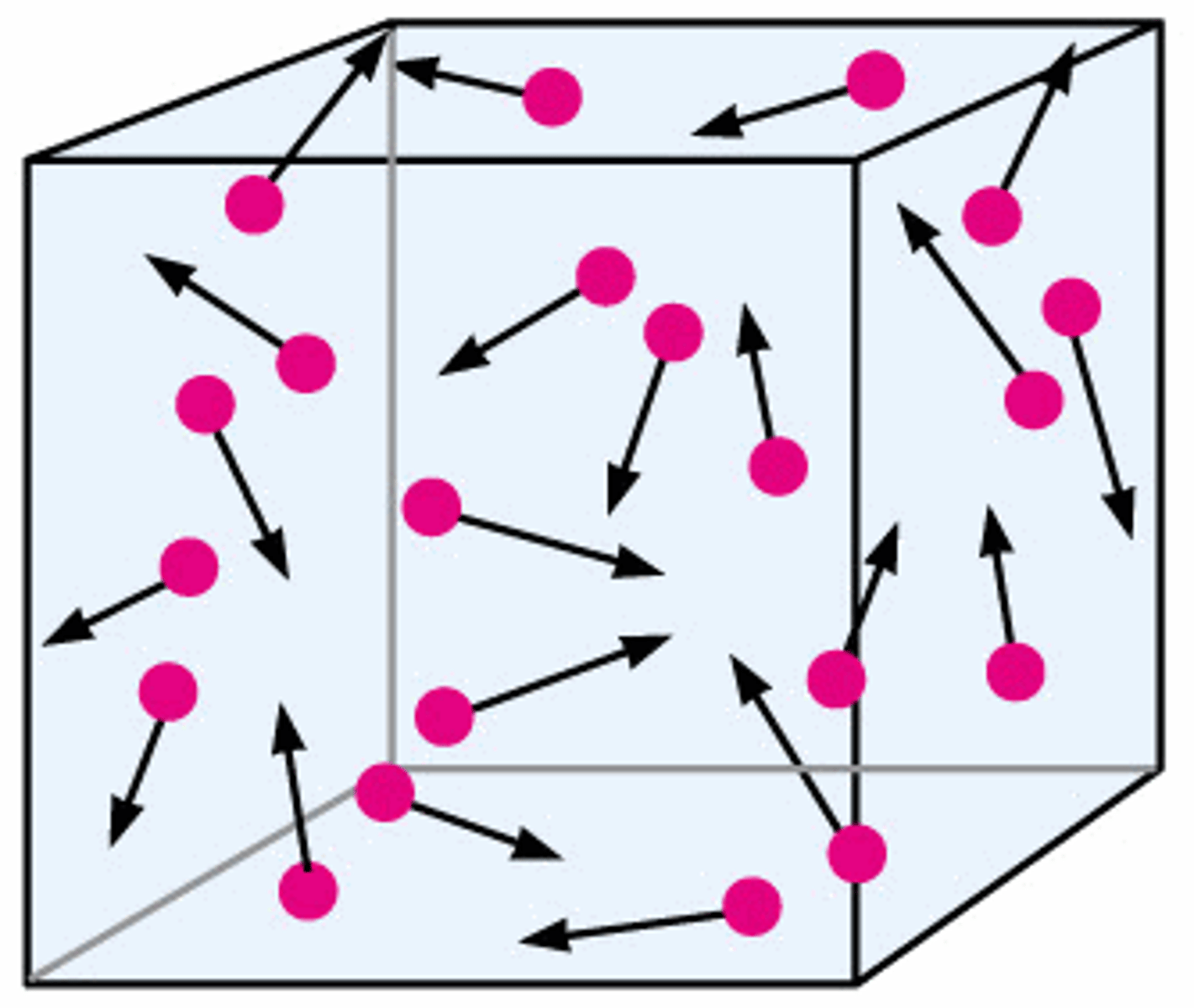
What is the Brownian motion?
collisions of rapidly moving molecules due to light
What is soap?
fatty acid salt forming a colloid
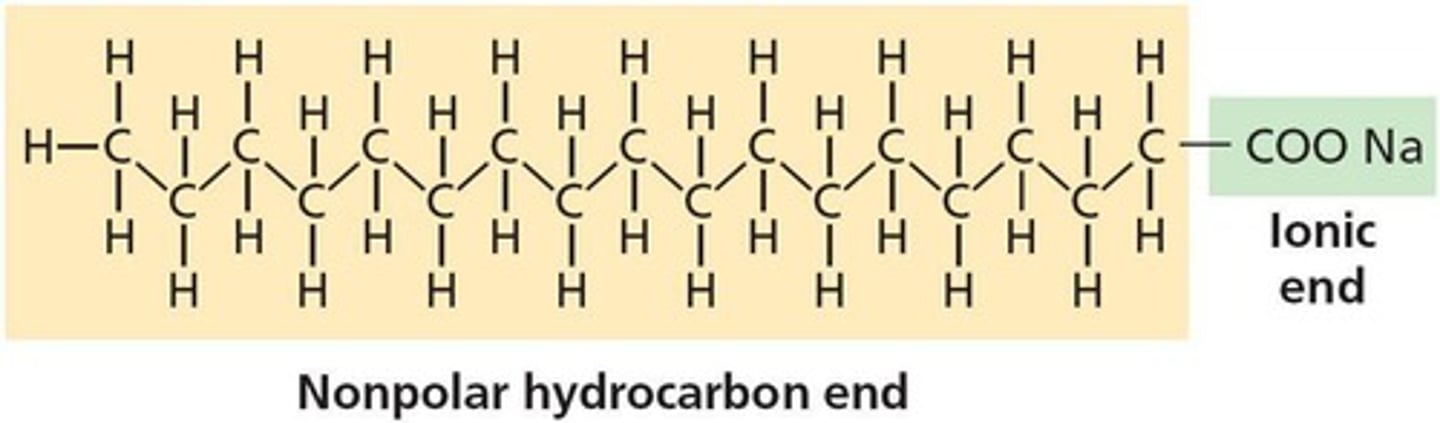
What is the structure of soap?
ionic head, nonpolar tail
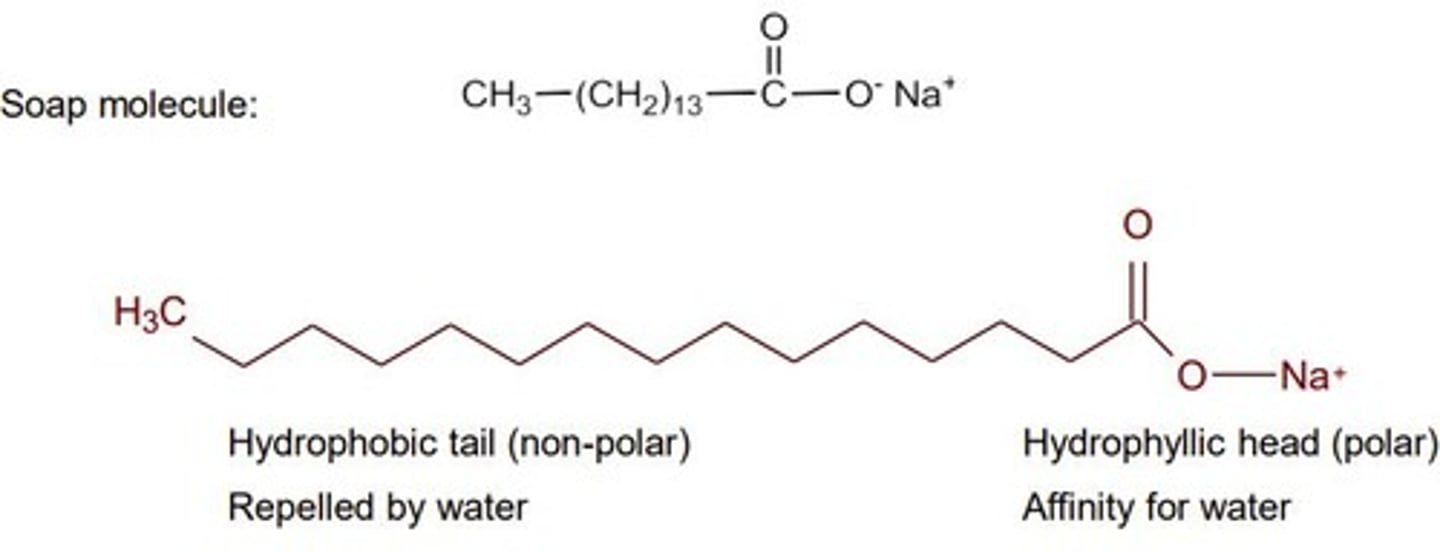
How do soaps help oily substances mix with water?
Head loves water (hydrophilic/polar), tail loves oil (nonpolar, hydrophobic) > forms micelles > mix oil + water