chemistry - groups in the periodic table: group 7 (6.6 - 6.13)
1/12
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
13 Terms
what are group 7 elements called?
halogens
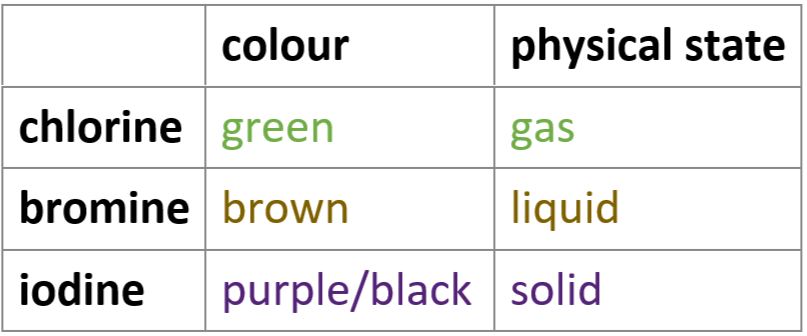
6.6 chlorine, bromine, iodine - colour & physical state at room temp.
6.7 chlorine, bromine, iodine - physical properties pattern
chlorine, bromine, iodine = increasing melting/boiling points
6.7 halogens - physical properties pattern (melting/boiling points)
melting/boiling points increase down group - top = lowest, bottom = highest
6.8 chemical test for chlorine
place damp blue litmus paper in chlorine gas
turns red then bleaches white
6.9 chlorine, bromine, iodine - reactions with metals
form metal halides:
e.g. chlorine + metal → metal chloride
6.9 halogens - reactions with metals pattern
halogen + metal → metal halide
6.10 chlorine, bromine, iodine - reactions with hydrogen then dissolved in water
form hydrogen halides
e.g. chlorine + hydrogen → hydrogen chloride
dissolve in water to form acidic solutions
e.g. hydrogen chloride + water → hydrochloric acid
6.10 halogens - reactions with hydrogen then dissolved in water pattern
halogen + hydrogen → hydrogen halide
hydrogen halide + water → hydro(halide) acid
6.11 chlorine, bromine, iodine - relative reactivity shown by displacement reactions with halide ions in aqueous solution
more reactive halogen displaces less reactive halogen from halide compound
e.g. chlorine + sodium bromide → bromine + sodium chloride
6.11 astatine - displacement reaction with halide ions in aqueous solution
bottom of group 7 = least reactive = won’t displace halide ion
6.12 displacement reactions - redox
redox reaction: one substance oxidised, one reduced
involve transfer of electrons - both oxidation & reduction occur at same time
e.g. chlorine + sodium bromide → bromine + sodium chloride
chlorine atom → chloride ion (reduction - gains electron)
bromide ion → bromine atom (oxidation - loses electron)
6.13 explain halogens relative reactivity (electronic configuration)
7 valence electrons - gain 1 electron when react
down group:
number of electron shells increases
force of attraction between + nucleus & - valence electron decreases
shielding increases
harder to gain electron
harder to form ions
reactivity decreases