Unit 2: Homeostasis / Water
3.0(3)
Card Sorting
1/29
There's no tags or description
Looks like no tags are added yet.
Last updated 12:24 PM on 9/15/22
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
30 Terms
1
New cards
Acid
A solution that measures less than 7 on the pH scale
2
New cards
Base
A solution that measures more than 7 on the pH scale
3
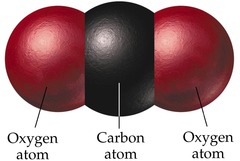
New cards
External
the outer surface of something (in this case, the body)
4
New cards
Feedback regulation
A control mechanism that uses the end result to regulate the rate of a process
5
New cards
Homeostasis
internal stability or "steady state" maintained by the body
6
New cards
Internal
the inner surface of something (in this case, the body)
7
New cards
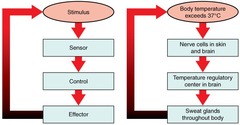
Negative feedback
A type of regulation that responds to a change in conditions by initiating responses that counteract or reverse the change
8
New cards
pH scale
The range of numbers used to describe how acidic a solution is

9
New cards
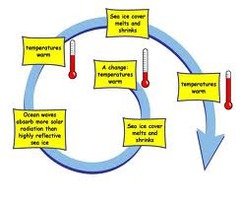
Positive feedback
A response to change when the stimulus increases the original stimulus
10
New cards
Response
A reaction (to a stimulus)
11
New cards
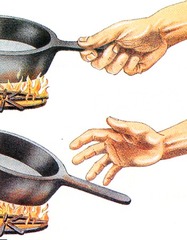
Stimulus
An environmental change that triggers a response
12
New cards
Adhesion
attraction between unlike molecules

13
New cards
Atom
smallest particle of an element
14
New cards
Cohesion
tendency of molecules of the same kind to stick to one another

15
New cards
Compound
substance containing two or more elements chemically combined in a fixed ratio
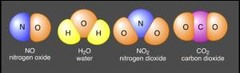
16
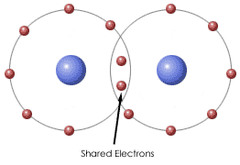
New cards
Covalent bond
chemical bond that forms when two atoms share electrons
17
New cards
Electron
subatomic particle with a single unit of negative charge
18
New cards
Hydrogen bond
bond created by the weak attraction of a slightly positive hydrogen atom to a slightly negative portion of another molecule
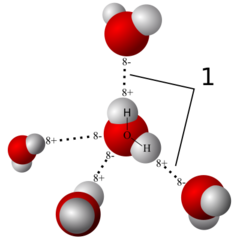
19
New cards
Ion
Atom that has become electrically charged as a result of gaining or losing electrons
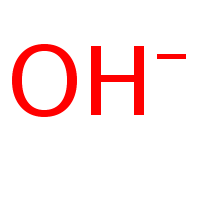
20
New cards
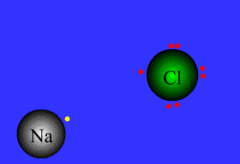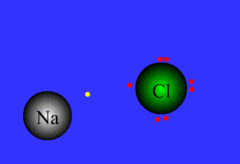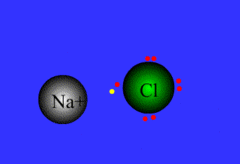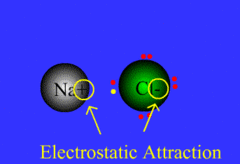
Ionic bond
Chemical bond that occurs when an atom transfers an electron to another atom
21
New cards
Molecule
two or more atoms held together by covalent bonds
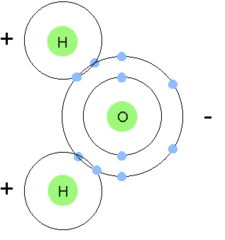
22
New cards
Neutron
subatomic particle that has no charge (electrically neutral)
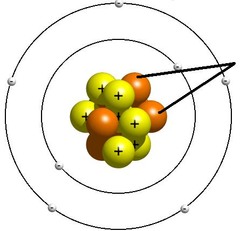
23
New cards
Nucleus (of an atom)
The central core that contains protons and neutrons
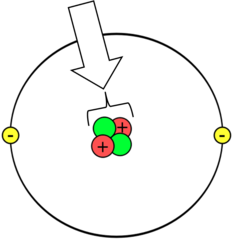
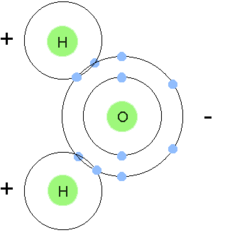
24
New cards
Polar molecule
molecule in which opposite ends have opposite charges
25
New cards
Proton
subatomic particle with a single unit of positive electric charges
26
New cards
Solute
substance in a solution that is dissolved and is present in a lesser amount

27
New cards
Solution
uniform mixture of two or more substances
28
New cards
Solvent
substance in a solution that dissolves the other substance and is present in greater amount

29
New cards
surface tension
A measure of how difficult it is to stretch or break the surface of a liquid

30
New cards
nonpolar
equal sharing of electrons