Drug Selection
1/35
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
36 Terms
What are factors to consider when selecting a molecule for further development?
Receptor activity and selectivity
Biological issues
Routes of administration
Metabolism issues
Physics-chemical issues
Ionisation potential (pKa)
Solubility (logP)
Biopharmaceutics Classification System (BSC)
Lipinski’s Rule of 5
Formulatability
Chemical issues
Synthesis
Stability
Patentability
Receptor Activity and Selectivity
Things to consider
Why are these things important to consider?
What is the target receptor?
What is the:
Emax (maximumum efficacy of the drug)
EC50 (conc at which 50% of Emax is reached)
IC50 (conc at which there is 50% inhibition)
Effect on other receptors:
Side effects
‘Dirty drugs’ e.g. haloperidol which affect many different receptors
Allows us to:
Achieve the target biological effect
Calculate the dose correctly
Predict side effects
Predict possible interactions and contra-indications
Explain what a receptor agonist and antagonist are
Receptor agonist: stimulates a receptor to promote the normal biological activity resulting from binding of the endogenous ligand.
Receptor antagonist: It binds to the receptor, but does not activate it. It "blocks" the action of the endogenous ligand or other agonists
Why is important to understand how a drug works in the body
Target biological effect can be achieved
Correct dose can be calculated
Potential side-effects can be predicted
Potential drug interactions and contra-indications can be predicted

You need to select a compound for development as a new anti-hypertensive, you have been given some data from your basic discovery colleagues, as shown.
Which one of the receptor activities will give the anti-hypertensive effect?
What side-effects are likely to be experienced by the patient?
What factors do you need to consider when evaluating these drugs? Explain your answer.
Which drug would you take forward for further development (if any)? Explain your answer.
α1 antagonist: causes vasodilation (not very specific - several side effects)
β2 antagonist: causes vasoconstriction
β1 antagonist: cardioselective, decreases heart rate and workload (more specific than α1)
α1 antagonist: α1 receptor stimulation causes smooth muscle vasoconstriction » inhibition causes reduction in bp
β2 antagonist: β2 receptor stimulation causes smooth muscle relaxation e.g. bronchodilation » inhibition causes bronchoconstriction
β1 antagonist: β1 receptor stimulation increases cardiac output, heart rate and blood pressure » inhibition causes reduction in bp
Potency on the target receptor: The most potent is Compound C (lowest IC50) and the least potent is Compound B (highest IC50)
Ratio between β1 and β2 activity: A greater separation means that off-target effects would be reduced at the clinical anti-hypertensive dose. Compound B is the best (ratio 1:25). Compound C is the worse (ratio 1:2)
Ratio between β1 and α1 activity: A greater separation means that off-target effects would be reduced at the clinical anti-hypertensive dose. Compound C is the best (ratio 1:25,000). This is 1:250 for A & B.
Describe the five modes of absorption of drugs across the intestine mucosa. What are the properties of the drugs that are processed in each route?
What are the factors mitigating against oral drug absorption? How do they work? What are the problems with this?
You have a range of compounds that you are studying. With respect to the effect of the mitigating factors discussed in Question 4, what would happen to the blood levels of your new compound in the following scenarios?
(A) Passive diffusion. Small non-ionized drug, in aqueous solution.
(B) Active transport. Drug needs to resemble natural molecules.
(C) Paracellular transport. Small and highly hydrophilic drug.
(D) Micellar delivery. Lipophilic drug.
(E) Via the immune system. Drug targeting the immune system
What are the factors mitigating against oral drug absorption? How do they work? What are the problems with this?
After an oral drug enters the gut lumen, it must cross intestinal epithelial cells to reach the blood. Two major systems reduce how much drug gets into the circulation:
P-glycoprotein (P-gp)
A membrane efflux pump in intestinal epithelial cells
Pumps drug molecules back into the gut lumen
Reduces net absorption
CYP3A4
A drug-metabolising enzyme in enterocytes
Metabolises drugs before they reach systemic circulation
Causes first-pass metabolism
Together, they act as a double barrier: even if a drug enters the cell, it may be metabolised (CYP3A4) or pumped out (P-gp).
How can CYP3A4 and P-gp explain drug-drug and drug-food interactions
Both CYP3A4 and P-gp can be induced or inhibited by other drugs or foods:
Induction → more enzyme/pump → less absorption
Inhibition → less enzyme/pump → greater absorption
Common drug and food interactions
Rifampicin » induces CYP3A4
Phenytoin » induces PGP
Ketoconazole » inhibits both PGP and CYP3A4
Grapefruit juice » inhibits CYP3A4
In each scenario, what would happen to the blood levels of your new compound in the following scenario:
a) Your patient is stabilised on your new orally-delivered compound, Compound D (metabolised by CYP 3A4). The patient is then given oral rifampicin as well as Compound D.
b) Your patient is stabilised on your new orally-delivered compound, Compound E(processed by PGP). The patient is then given oral phenytoin as well as Compound E.
c) Your patient is stabilised on your new orally-delivered compound, Compound F (processed by PGP and metabolised by CYP 3A4). The patient is then given oral ketoconazole as well as Compound F.
d) Your patient is stabilised on your new orally-delivered compound, Compound G (not processed by PGP and not metabolised by CYP 3A4). The patient then makes a change to his diet (to try to be more healthy) and starts to take his medicine at breakfast with grapefruit juice.
a)
↑ CYP3A4 activity
↑ intestinal metabolism
↓ drug reaching blood
Net effect: Lower absorption in blood
b)
↑ efflux back into gut lumen
↓ net uptake
Net effect: Lower absorption in blood
c)
↓ metabolism
↓ efflux
More drug crosses into bloodstream
Net effect: Greater absorption in blood
d)
↓ first-pass metabolism in gut
↑ systemic exposure
Net effect: Increased absorption / higher blood levels
Explain the important of logP of a drug in its oral absorption
logP
The ratio of the concentrations of a (non-ionised) solute between two solvents
If one of the solvents is water and the other is a non-polar solvent, log P becomes a measure of hydrophobicity (or lipophilicity)
logP < 0 (more hydrophilic)
logP > 0 (more hydrophobic)
logP = 0 (same affinity for both phases)
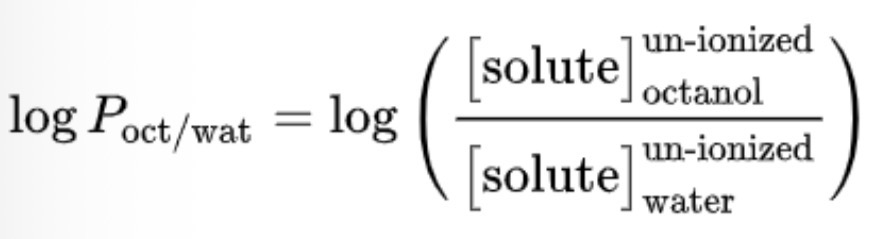
Explain the important of pKa of a drug in its oral absorption
Provides information on how much drug is in the ionised or un-ionised state, at various pH conditions
pKa
Acid Dissociation Constant

pKa
Base Dissociation Constant

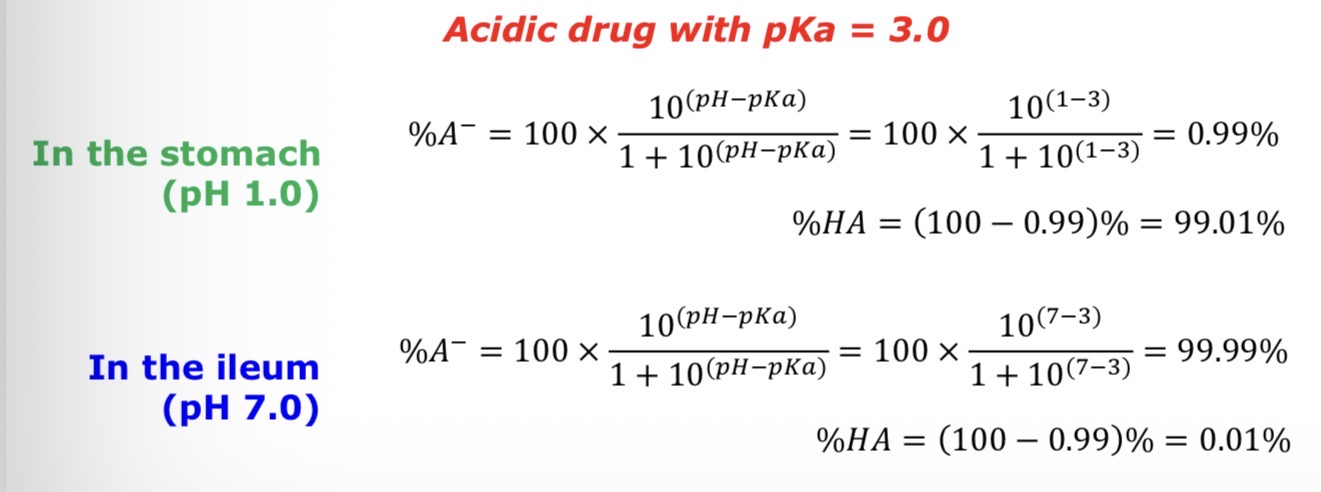
How much is in the ionised and unionised state in the stomach (pH = 1.0) and terminal ileum (pH = 7.0) for the following drug:
Acidic drug with pKa = 3.0
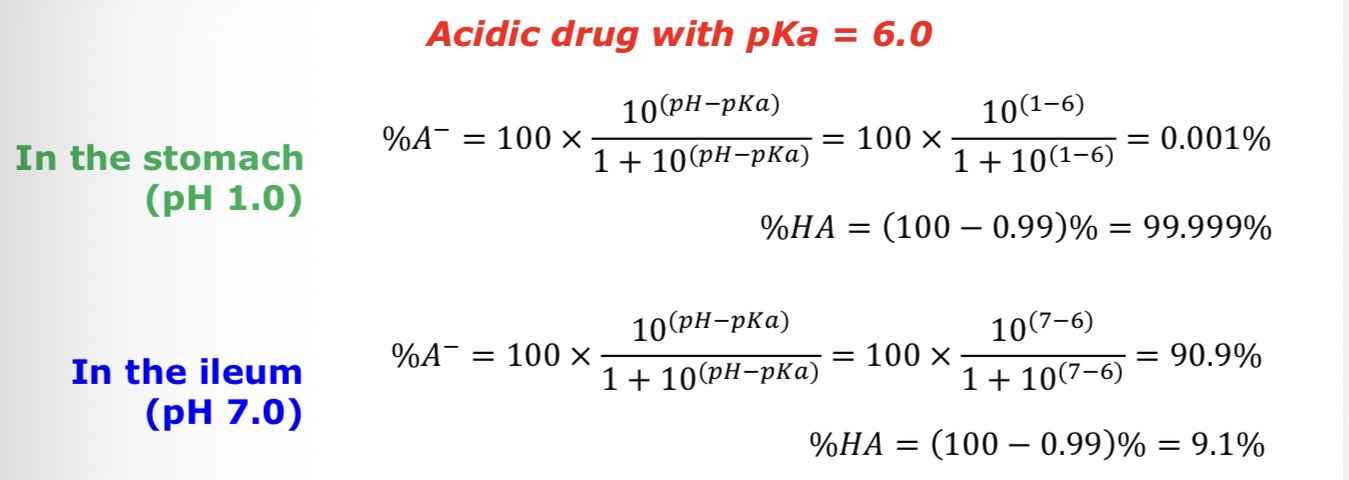
How much is in the ionised and unionised state in the stomach (pH = 1.0) and terminal ileum (pH = 7.0) for the following drug:
Acidic drug with pKa = 6.0
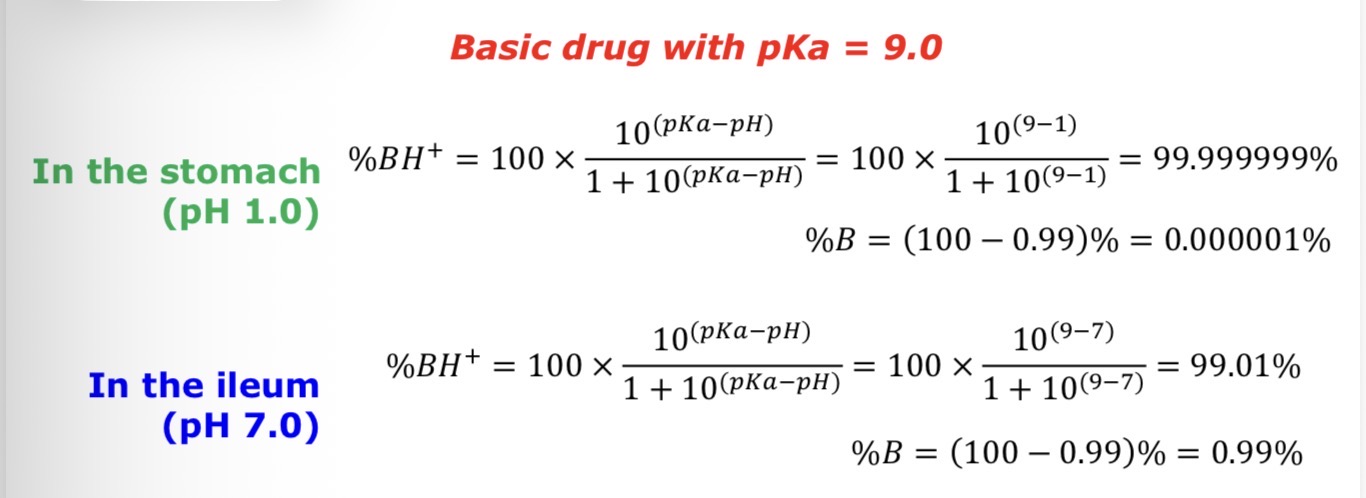
How much is in the ionised and unionised state in the stomach (pH = 1.0) and terminal ileum (pH = 7.0) for the following drug:
Basic drug with pKa - 9.0
How much is in the ionised and unionised state in the stomach (pH = 1.0) and terminal ileum (pH = 7.0) for the following drug:
Basic drug with pKa = 12.0

What is the Biopharmaceutics Classifications System (BCS)
Classifies all drugs into 1 of 4 classes
state all the classes in BCS
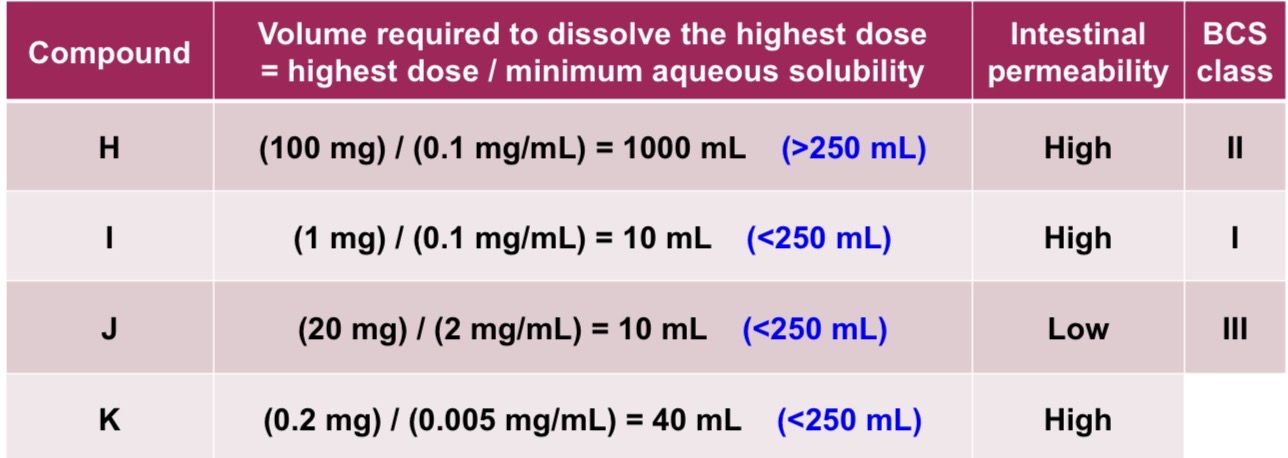
Class 1: High solubility, high permeability
Class 2: Low solubility, high permeability
Class 3: High solubility, low permeability
Class 4: Low solubility, low permeability
What BCS class is the most favourable?
Class 1: High solubility, High permeability
Has the highest bioavailability
What does high solubility refer to?
Highest dose is soluble in 250mL or less of aqueous medium with a pH of 1-6.8
What does high permeability refer to?
No less than 85% of the administered dose is expected to be absorbed
Application of BCS
Developing formulations of a drug
If drug is class 1
Dissolves rapidly and rapidly absorbed across the gut
So good bioavailability
So any formulation can be used
If drug is class 2
Dissolution rate is likely to limit absorption
So use formulations that enhance dissolution rate
If drug is class 3
Dissolves rapidly but poorly permeable
So formulation used needs to release drug rapidly to maximise the time the drugs have to permeate the GI tract
If drug is class 4
Very poor bioavailability
Oral route might be impossible so we need to consider a prodrug
What is a prodrug?
inactive or less active form of a drug that is metabolized in the body to produce its active form
What is the BCS-based biowaiver?
Approves generic drugs
By doing bioequivalence studies using the BCS
A way to compare 2 drugs without human studies
What is bioequivalence?
When the two drug products (generic vs branded) release the same amount of active ingredient into the bloodstream at the same rate and extent when given at the same molar dose
This ensures that a generic drug works the same way as the original branded drug
Which class of drugs may be able to be licensed without the need for a BCS-based waiver?
Class 1
Class 3

For the following compounds, estimate their BCS classes based on the information given
Explain Lipinki’s ‘Rule of 5’ and why it is important in compound selection
A rule to determine if a pharmaceutical compound has chemical and physical properties that would make it an orally active drug
It can be used during drug discovery to optimise activity and selectivity of a pharmaceutical compound
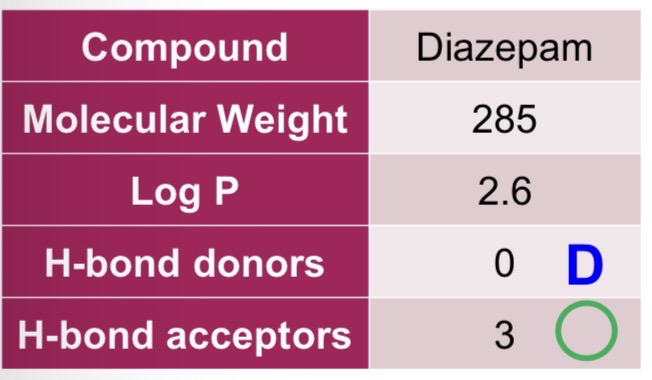
Rule of 5:
Molecular weight ≤ 500 - greater solubility, enhances diffusion
Log P ≤ 5 - greater absorption
H-bond donors ≤ 5 - greater membrane partition and permeability
H-bond acceptors ≤ 10 - greater membrane partition and permeability
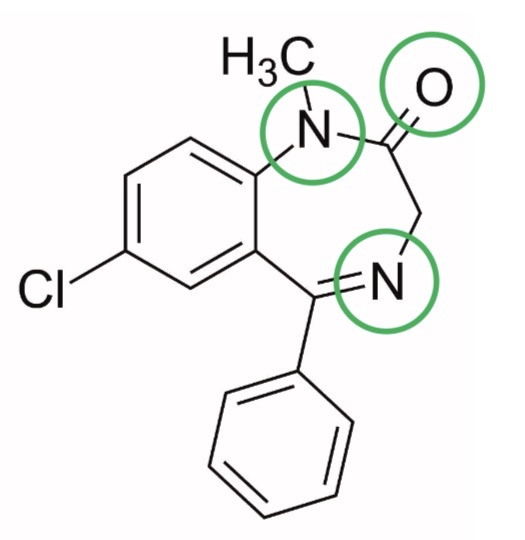
For the following drug, state whether it complies with the Rule of 5:
Diazepam
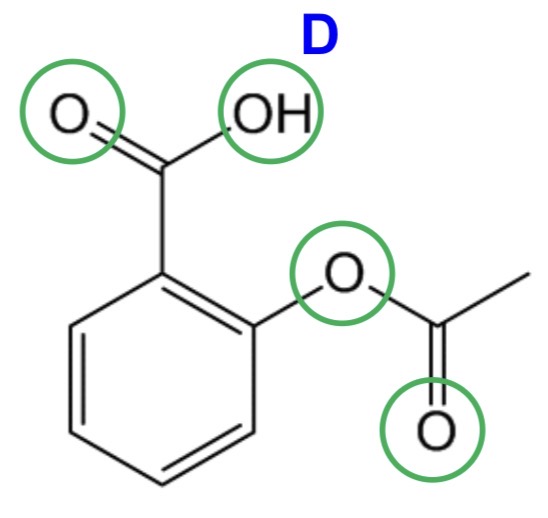
For the following drug, state whether it complies with the Rule of 5:
Aspirin
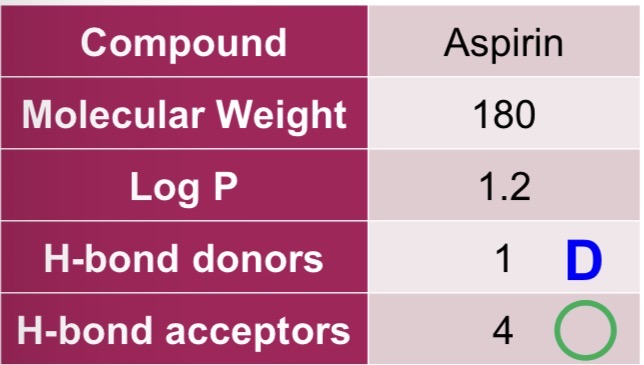
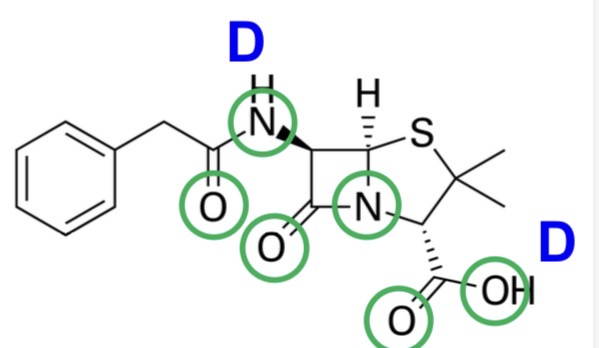
For the following drug, state whether it complies with the Rule of 5:
Benzylpenicillin
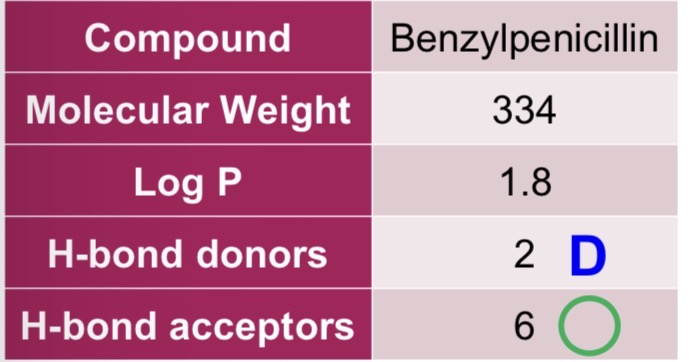
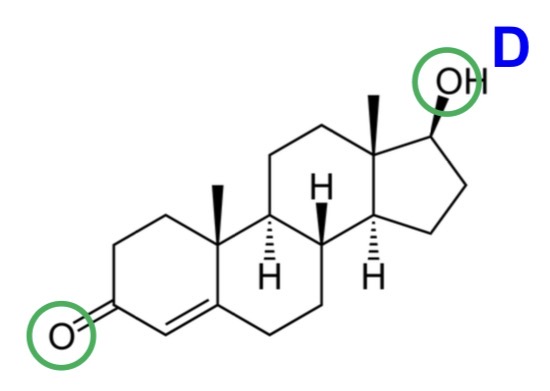
For the following drug, state whether it complies with the Rule of 5:
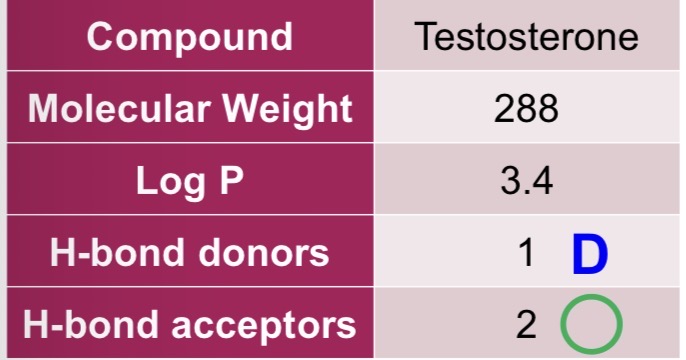
Testosterone

Given the following information on their chemical synthesis, which compound would you choose to take forward as a development candidate. Explain your answer
Compare number of steps
Compound M has the lowest number of steps
Calculate overall yield for each compound (yield number of steps):
Compound L: Overall yield = 0.755 = 0.24 = 24%
Compound M: Overall yield = 0.74 = 0.24 = 24%
Compound N: Overall yield = 0.96 = 0.53 = 53%
High temperatures?
Compound M and N dont use high temperatures
Cost
Compound L has the lowest cost, Compound M has medium cost
Compound N is the best development candidate:
Highest overall yield by far → less starting material, less waste
No high-temperature steps → safer, easier to scale
Medium cost per step, offset by much better yield
Despite having more steps, the high per-step efficiency dominates
How long is a patent term?
How long is the development time?
20 years from first registration
About 10 tears