Nucleophiles, Electrophiles and Leaving Groups
1/37
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
38 Terms
Almost all organic reactions can be divided into two groups. What are they?
Oxidation-reduction
Nucleophile-electrophile
Nucleophiles and electrophiles are particularly important to reactions involving which two functional groups?
Alcohols and Carbonyl containing compounds
Nucleophile
Lone pair or pi bonds
which can form new bonds with electrophiles
Nucleophiles as they relate to bases
Good nucleophiles tend to be good bases
How are nucleophiles and bases different?
How they are measured
Nucleophiles strength is based on relative rates of reaction with a common electrophile
Base strength is measured by equilibrium position of a reaction
What are some common nucleophiles?
Anions: Br-, OH -, CN-
Pi bonds: Alkene, Alkyne, Benzene
Atoms w/ Lone Pairs: water, NH3, Acetone
What four factors determine nucleophilicity ?
Charge: more charge = stronger
Electronegative: less electronegative = stronger
Steric hindrance = smaller = stronger
Solvent = aprotic = stronger
Why is aprotic better for nucleophile strength?
Because no hydrogen bonds means no protonation of nucleophile or intermolecular forces with nucleophile to decrease it's strength
Why are less electronegative atoms better for nucleophile strength?
Because they are less likely to hold onto the electron density meaning that they are more likely to give it up, thus react with electrophilic.
What's the trend for nucleophilicity for POLAR protic vs POLAR aprotic solvents?
Nucleophilicity increases down the table for protic solvent
Nucleophilicity increases up the table for aprotic solvents
Common protic solvents
Carboxylic acids
Ammonia/amines
Water
Alcohols
Common aprotic solvents
Dimethyformamide (DMF)
Dimethylsulfoxide (DMSO)
Acetone
Dimethylsulfoxide (DMSO) structure

Dimethyformamide (DMF) structure
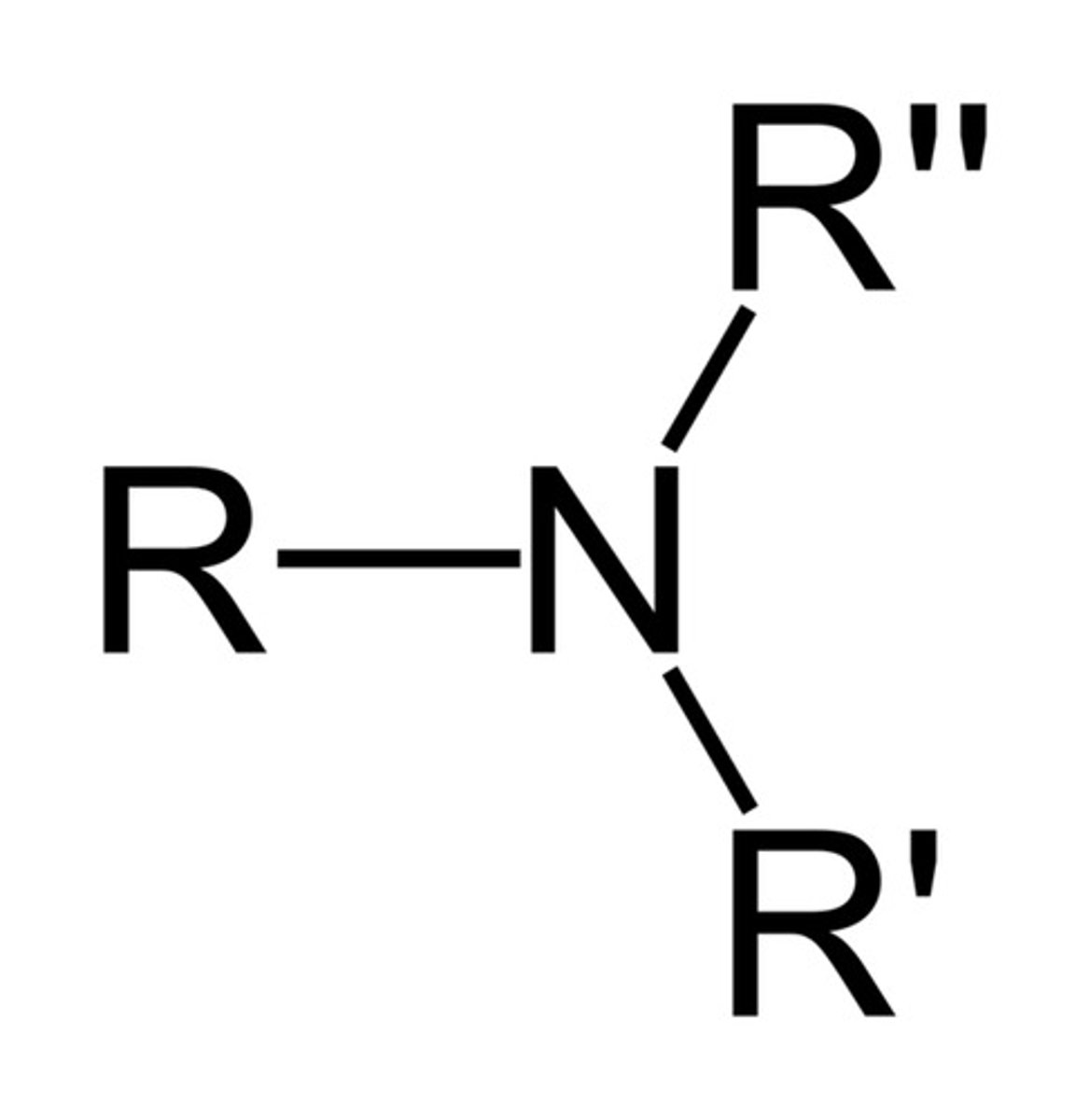
Amine structure
Can be 1, 2, OR 3
Acetone (structure)
most basic ketone = acetone

If solvent isn't given on test day, what do should you assume?
It is polar solvent
polar solvents, whether protic or aprotic can assist in movement of electrons (WHICH IS WHAT OCHEM is all about)
I>Br >Cl > F
Nucleophilicity for which type of polar solvent? Why?
Polar protic solvent
HF = weak acid = unstable CB
thus F- is more likely to form bonds with protons than a strong acids CB which is more stable
HI = strong acid = stable CB
protons less likely to form bonds because the stable CB is less likely to be affected by the protons in solution. This allows it to act on the electrophile
F> Cl> Br > I>
Nucleophilicity for which type of polar solvent?
Polar aprotic
No protons in the solution means that they can't get in the way of the nucleophile
Thus aprotic solvents, nucleophilicity relates directly to basicity.
Examples of strong nucleophiles
OH-, RO-, CN-, N3-
Examples of Medium nucleophiles
Nh3 and RCO2- (ester ion)
Examples of Weak nucleophiles
H20, RCOOH, ROH
Electrophiles
positive charge or partially positive
accepts electron pair to form bonds with nucleophile
Difference between electrophilicity and lewis acid ?
Both are electron acceptors but they are measured differently
Electrophiles = kinetic property
Lewis Acid = thermodynamic property
Electrophilicity increases with ?
Positive charge
Why Carbocation is more electrophilic than Carbonyl atom
What can affect the electrophilicity of a species that has no empty orbitals?
The strength of leaving group
better leaving group makes reaction more likely to happen
If empty orbitals are present on the electrophile, what does this mean for the leaving group?
The nucleophile can form bond with the electrophile without kicking off leaving group
Electrophilicity and acidity are effectively identical properties when it comes to reactivity
Examples of good electrophiles
Alcohols, ketones, carboxylic acids, aldehydes and carboxylic acids derivatives
Why can't you use nonpolar solvent in a electrophile-nucleophile reaction?
Because the reactants are polar and need to be dissolved into solution
Nonpolar solvents wouldn't dissolve them
What's the order of best electrophilicity of the carboxylic acid derivatives
Anhydrides
Carboxylic acids
Esters
Amides
Leaving Groups
leaves and take the electrons with them
Heterolytic group = opposite of coordinate covalent formation
What's a characteristic of good leaving groups
able to stabilize the extra electrons after leaving with them
How are good leaving groups related to strong acids?
Strong acids are strong because the CB is stable and doesn't mind leaving
Good leaving groups are stable and don't mind leaving
What are some good leaving groups?
I-
Br-
Cl-
What can increase the strength of leaving group to leave?
Resonance
Inductive effect by electron withdrawing groups
Helps delocalize negative charge and stabilizes it.
What are two of the worst leaving groups, ones that will almost never act as leaving groups?
Alkanes and hydrogen ions
form very reactive, strongly basic anions
can think of leaving group and nucleophiles as serving opposite functions, how?
In substitution reactions weaker base acts as leaving group and is replaced by stronger base which is nucleophile