PHR 937 immunology
1/47
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
48 Terms
What is innate immunity?
immediate, nonspecific protection against foreign substances
What is adaptive immunity?
humoral and cellular immunity that is specific to the antigen
What is humoral immunity?
antibody-mediated (B-cell) immunity
What is cellular immunity?
T-cell immunity
When does adaptive immunity peak?
about 14 days
What are neutrophils?
white blood cells that are the early stage responder to infection; they phagocytose or degranulate, killing bacteria internally and externally
What is myeloperoxidase?
a neutrophil enzyme within granules that is responsible for breaking down foreign material
What are macrophages?
white blood cells that swallow infectious pathogens and foreign material left behind by neutrophils and lymphocytes; can also be antigen presenting
What are dendritic cells?
white blood cells that take surveillance of surrounding tissues; they migrate to LNs to present antigens, linking the innate and adaptive immune system
What are natural killer cells?
innate white blood cells that degranulate epitope specifically by attacking infected cells
Why are NK cells important for rituximab?
NK cells bind to rituximab on B-cell and cause degranulation of the B-cells.
What are mast cells?
white blood cells that contain granules of histamine; responsible for allergic reactions
What antibody is responsible for mast cell degranulation?
IgE
What are regulatory T cells?
type of T cell that is responsible for preventing overactivity of the immune response; to prevent autoimmunity
What is HLA?
human leukocyte antigen; peptide complex that is antigen presenting cell in humans
What are PAMPs?
pathogen associated molecular patterns
What are PAMPs and DAMPs recognized by?
pattern recognition receptors (PRRs)
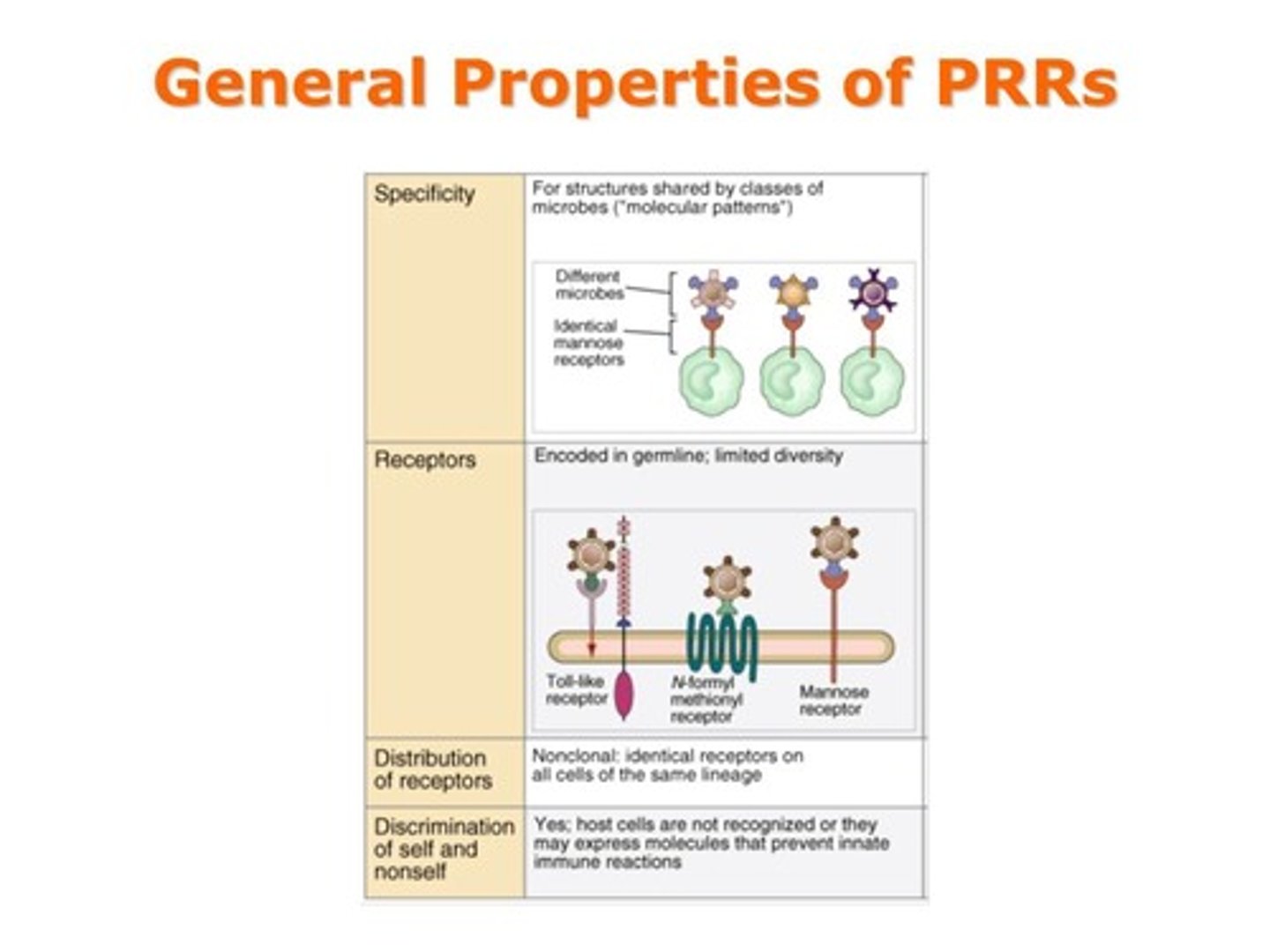
What are DAMPs?
damage associated molecular patterns
What is TL4?
toll-like receptor 4; binds LPS
What happens with LPS binds to TL4?
dimerization, recruitment of accessory proteins, and signaling to induce transcription factor expression
What soluble PRRs does the liver release upon activation by IL-6?
- SP-A and SP-D
- mannose binding lectin
- fibrinogen
- CRP
- serum amyloid protein
How are B cells activated?
B cell is activated when it encounters an antigen that matches its B cell receptors and receives cytokines from helper T cells.
What pathways can a B cell take when B cells proliferate?
- plasma cells: secrete antibodies
- IgG expressing B cells: isotype switching
- high affinity IgG expressing B cells: affinity maturation and memory B cell formation
What do plasma cells secrete?
IgM
What is the difference between IgM and IgG?
IgM has lower affinity and must bind together to create IgM complexes to bind more proteins; IgG has higher affinity for proteins and can neutralize more rapidly
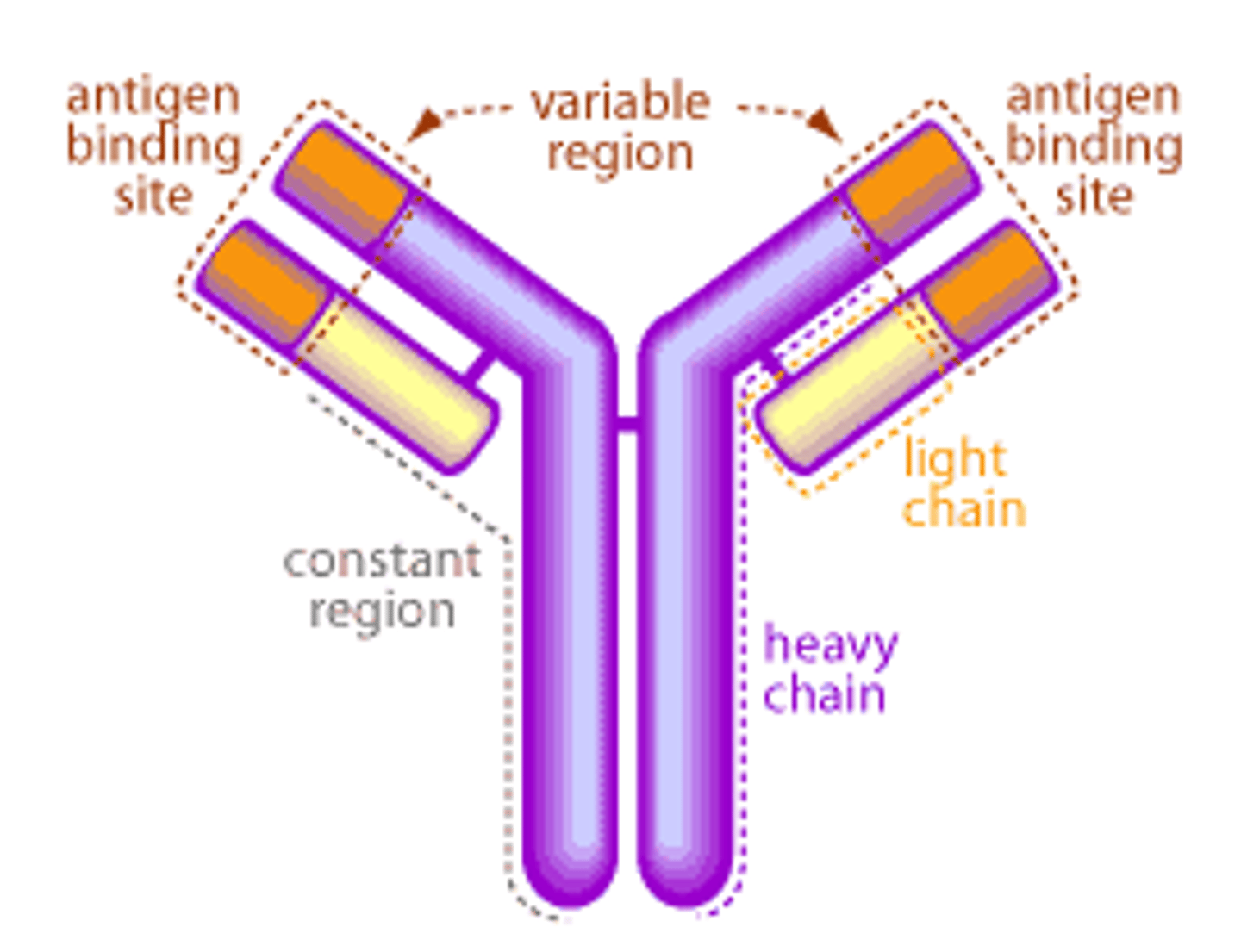
What is the structure of an antibody?
Y shaped molecule
-4 polypeptide chains: 2 heavy identical chains and 2 light identical chains held together by a disulfide bond
- 4 constant regions (C), and 4 variable regions (V)
- antigen binding sites
What isotype are most therapeutic antibodies?
IgG
What is affinity?
the strength of binding between a single binding site and a single ligand
What is avidity?
the strength of binding between a molecule and a complex ligand; avidity increased by increasing the number of binding sites or by increasing the affinity of those binding sites
What types of proteins may not look like self?
post-translation modified proteins; ex: CCP
What are thymus-independent antigens?
antigens lacking peptide components, making them unable to be presented to T cells
What are thymus-dependent antigens?
antigens containing peptide components
What do T cells bind?
chopped up antigens made of protein
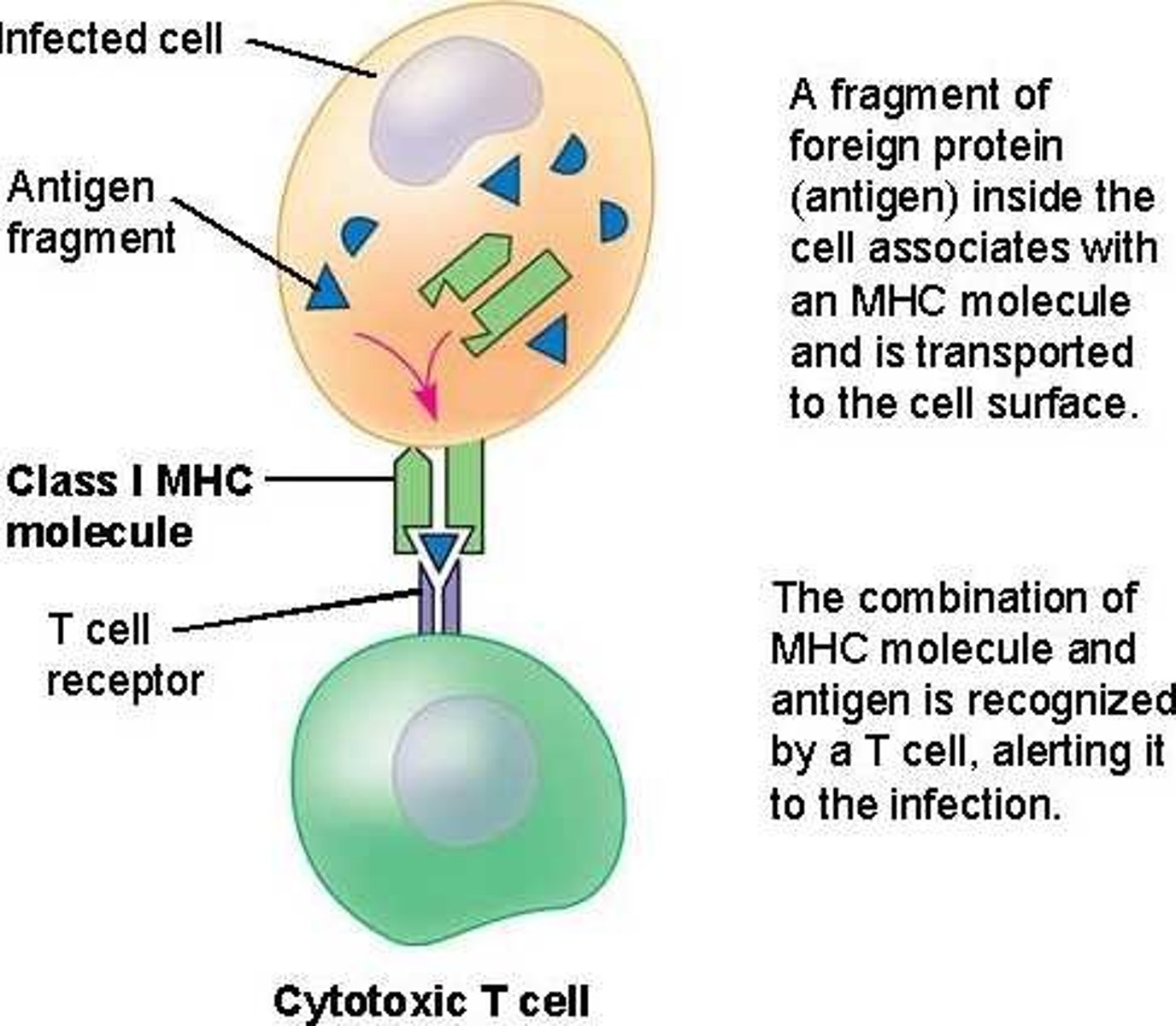
What is MHC I? What binds to it?
major histocompatibility complex I
CD8 binds to them
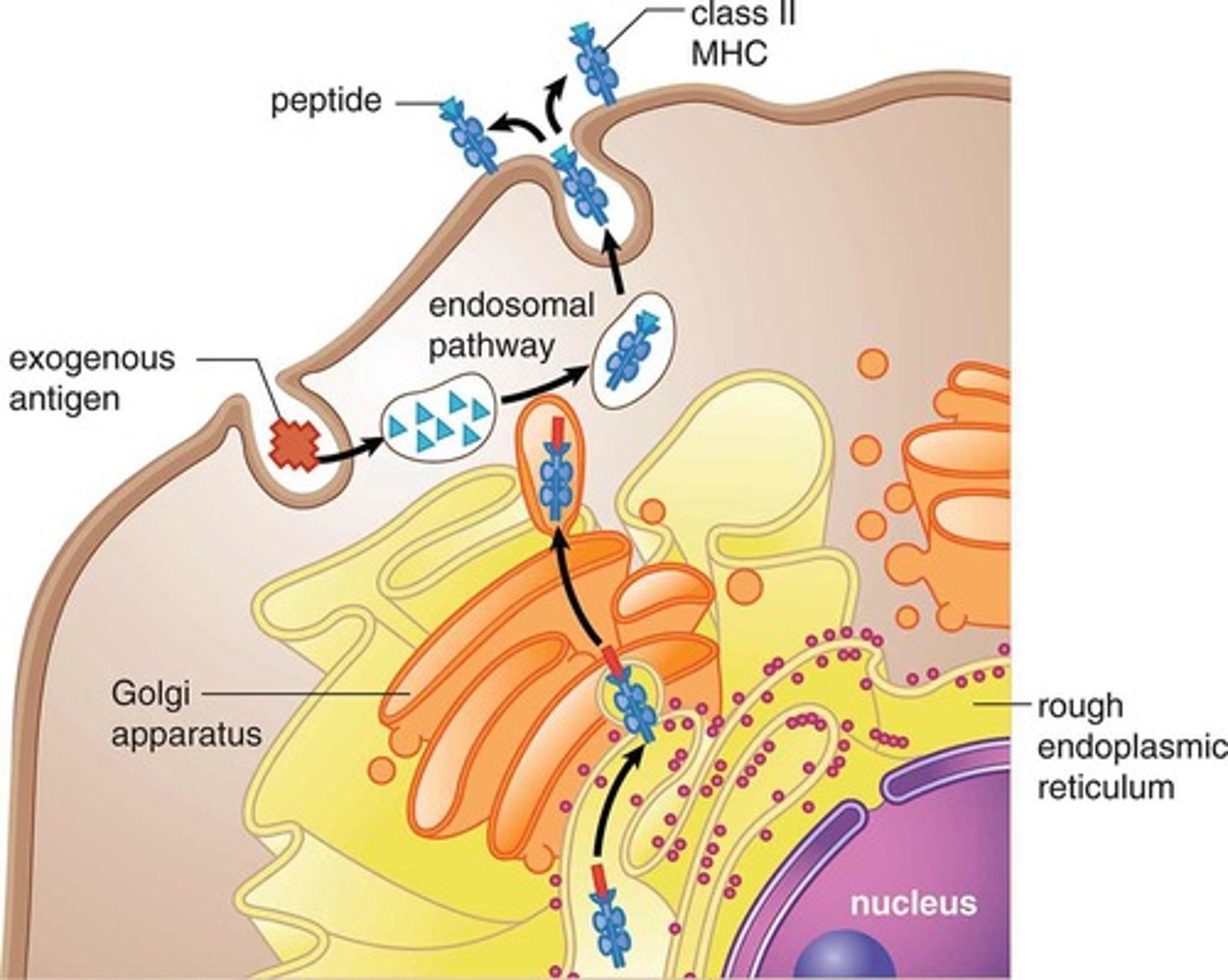
What is MHC II? What binds to it?
major histocompatibility complex II
CD4 binds to them
Which cells in the body have MHC I?
all cells in the body; important bc need to be able to be attacked by killer T cell in case of infection within cell
Which cells in the body have MHC II?
APCs
What is the purpose of helper T cells (CD4)?
enhance B cell activity and affinity
Why do B cells need help from T cells?
helper T cells interact with B cells to activate them and initiate more specific antibody production
What do costimulatory inhibitor therapeutics do?
they prevent T-cells from helping B-cells
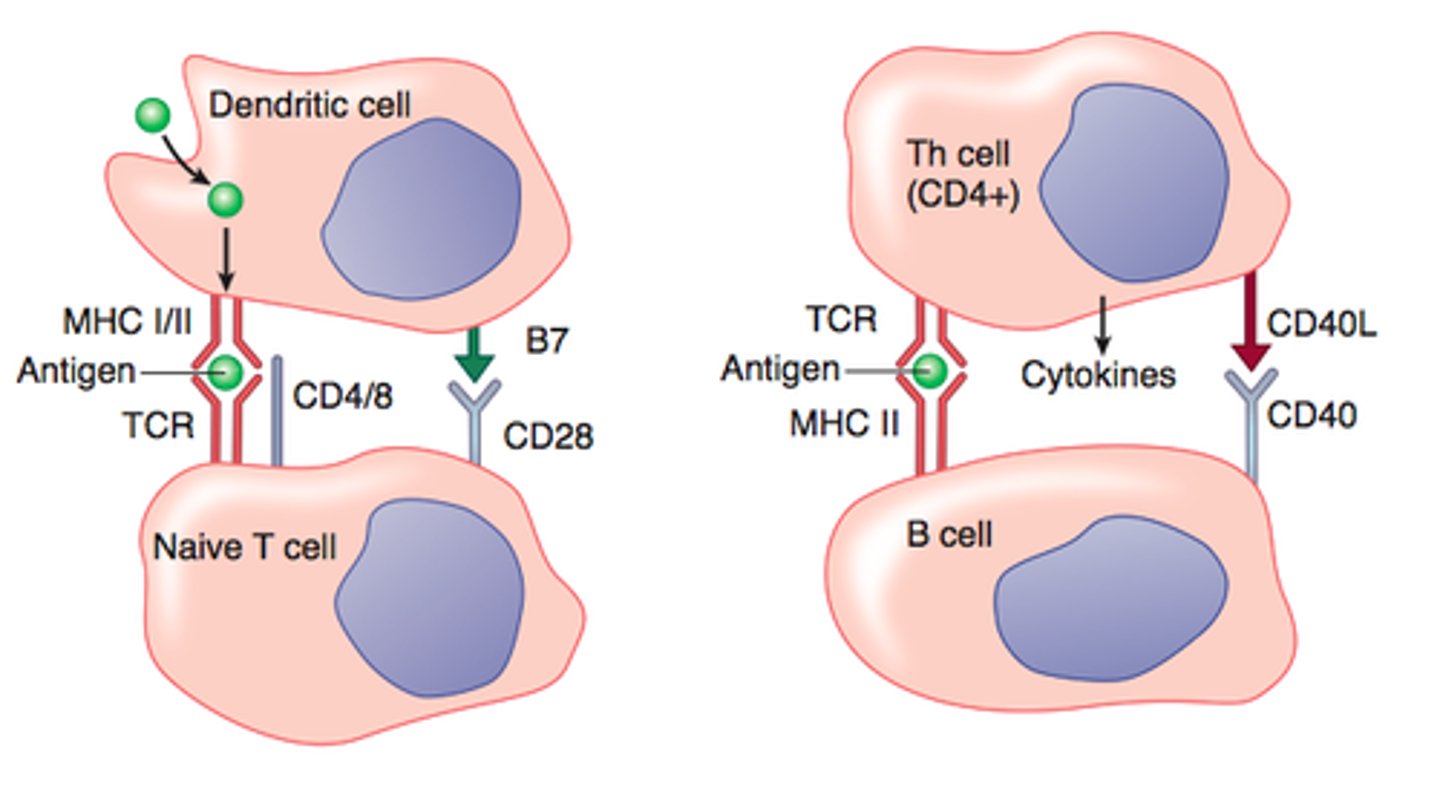
What do you need for T and B cells to release cytokines?
peptide MHC and costimulatory molecules
What is the purpose of pegolation of an antibody?
it allows it to stay in the circulation longer
What is FcRn?
neonatal Fc receptor; expressed by endothelial cells and circulating monocytes. It extends the serum half-life of antibodies because it prevents the lysosmal degradation by binding them in the endosomal compartment and "shuttling" them back into the blood stream.
What are the methods of neutralization of a pathogen?
- neutralization of microbes and toxins
- opsonization and phagocytosis of microbes
- antibody-dependent cellular cytotoxicity (NK cells)
- phagocytosis of microbes opsonized with complement fragments
- inflammation and lysis of microbes after complement activation
What is complement activation?
antibody activates complement, which enhances opsonization and lyses some bacteria
What is positive/negative selection in the thymus?
lymphocytes are either neglected or undergo apoptosis due to high affinity for self; those who have the right affinity are then positively selected and exported into periphery
Why is positive/negative selection important?
If it is broken, lymphocytes with high affinity for self go into circulation
What mechanisms lead to autoimmunity?
1. escape from negative selection
2. post-translational modifications
3. loss of regulatory t cells
4. epitope similarity with infection