ENGINEERING TOPIC 1
1/29
Earn XP
Description and Tags
chingk kchong
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
30 Terms
X-ray Testing (non-destructive)
Involves passing x-rays through an object and exposing a photographic film on the opposite side
Dye Penetrant Testing (non-destructive)
dye is placed over the surface with the excess wiped off, SHOWS CRACKS
Ultrasonic Testing (non-destructive)
Transmitter sends ultrasonic waves through an object and reflected back to the transmitter - These reflected signals are recorded on a display, where the height of the signal is proportional to the thickness of the object (cavities will show a lower reading)
Transverse Testing (destructive)
Also known as a three point bend test
- Material is supported at either end and a load is applied on in the centre between the two supports, or at positions two and two thirds between the supports
- A measure is taken of the bending load and total deflection at rupture, which can be converted into stress and strain
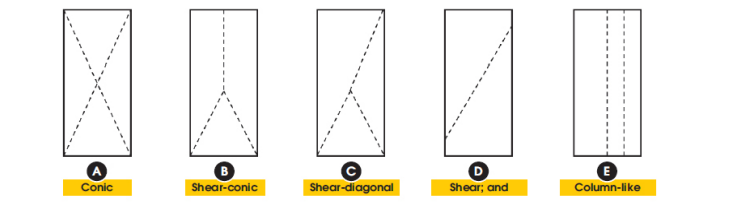
Compressive Testing - Concrete testing
For concrete, compressive testing will be done at specified time intervals to ensure a proper mix
- Usually, concrete fails in hourglass fractures or a shear fracture but can also can fracture in other ways
Slump Test - Concrete testing
Slump test is used to check that the concrete is of appropriate fluidity for casting
- The concrete is cast into a conical mould, where the top and bottom are open.
- Upon removing the mould, the concrete should slump slightly. If it collapses, it is too wet and if it breaks/crumbles it is too dry
Strain Energy - Crack theory
When a material is deformed and worked, it gains strain energy
The cracking of a material is the release of its strain energy and has an impact on the formation and propagation of cracks
Crack Formation and Growth - Crack theory
Crack formation and growth is a mechanism of brittle failure. A metal alloy may undergo ductile failure up to a point, increasing in length, until it cracks from brittle failure
- Cracks start when there is an imperfection in the atomic planes of an object, i.e. a bond may be missing
- This will concentrate twice the stress at the next bond which can lead to its failure, then placing more stress at the next bond, and so on (leads to the formation of a crack)
- Strain energy can be concentrated at tips of cracks and sharp points, so when cracks grow larger they grow faster and faster (sharp points should be eliminated with fillet curves)
Critical Crack Length - Crack theory
When a specific crack length is exceeded under the load conditions that created it, the crack will travel through the material until failure occurs
- The longer the critical crack length of the material, the less likely it is to fail under brittle mechanisms (critical crack length is proportional to the Young’s Modulus of a material)
Crack Repairs / Elimination - Crack theory
Repairing metal alloys through welding may weaken the material (a point of stress concentration) thus are heat treated to avoid such problems
In polymeric materials, it can be possible to use adhesives to repair cracks. Furthermore, for thermoplastics it can be possible to weld the polymer which offers strengths close to the parent material
To prevent crack formation, an object should be designed without sharp corners which tend to concentrate stress
An interface can also be used to prevent cracks, which is an area within a material is weaker than the surrounding area and runs perpendicular to the expected path of the crack
When a crack reaches the interface it is unable to pass and thus prevents it from getting to the critical crack length
Stone - Ceramics
All stones exhibit similar mechanical properties. It is weak in tension, strong in compression and has low toughness (brittle)
- Furthermore, stone shows good wear resistance but is labour intensive to work with
- Basalt, which is an igneous rock, is used in concrete aggregate
Glass - Ceramics
- Amorphous solid, meaning it has no regular crystalline structure and is brittle due to the limited slip planes (weak in tension and shows perfect brittle fracture)
- Toughened or tempered glass has been heated so that the outer surfaces are placed in compression, increasing the force required to place it in tension
- In laminated glass, a polymer holds two layers of glass together which increases safety as shards are held together when shattered
- Glasses can also be chemically strengthened by adding foreign molecules to distort the structure of the glass and placing it into compression
Cement - Ceramics
Ceramic material formed when complex reactions with alumina, soda and lime occur in an exothermic reaction
- Hydraulic cements harden underwater whereas non-hydraulic cements do not
- Similar properties to stone (strong in compression, weak in tension, brittle) but has the advantage to be cast in almost any shape
- Cement (and concrete) dries hard within a day or two, but does not reach full strength for many years
Bricks - Ceramics
- Rectangular building blocks traditionally made of clay, but can also be formed from concrete
Formed by extrusion (holes) or pressing (solid), then fired in a kiln to set its permanent shape
- Large brick walls can be made of besser blocks, which are concrete blocks with two hollow channels
Timber - Composites
Natural composite of cellulose fibres held together by lignin (natural resin)
- Timber can be classified into softwoods and hardwoods, but the names are rather misleading
- Hardwoods come from flowering trees, softwoods come from evergreen trees (pines and conifers)
- Exhibits high strength to weight ratio and has reasonable performance in bending. However, it is susceptible to adverse weather conditions and pest attacks (termites)
Mortar - Composites
- Used between bricks in buildings and is a mixture of sand, cement and lime
Concrete - Composites
Composite of cement, sand and aggregate (commonly basalt) with a typical mixture of 4 parts aggregate, 2 parts sand and 1 part cement and wartA
- Sand fills gaps between the aggregate and the cement acts as the binder that holds them together
- Strong in compression, weak in tension and has low toughness. Also fireproof and corrosion resistant
- Water content of concrete is determined by two factors, workability and final strength. If the water content is too low, the concrete will not be fluid enough to cast. If there is too much water, it will flow well but the final strength will be low and shrinkage can become a problem.
Reinforced Concrete - Composites
- The low tensile strength of concrete can be mitigated by reinforcing it with rods or steel mesh
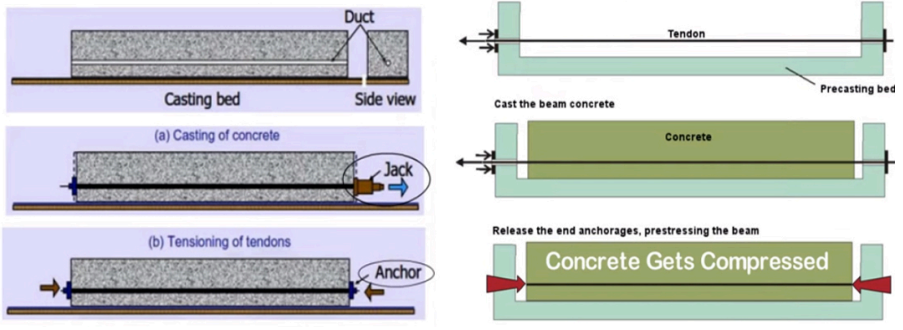
- Pre-tensioned reinforced concrete is created when the concrete is cast over a series of steel rods or cables that have been tensioned prior to pouring (economical and uniform + high quality but not done on site so easily)
- Post-tensioned concrete is formed when concrete is cast with tubes and when set, wires are pulled through the slab which are anchored to plates at one end and tensioned and left in tension (can be done on site but is more complex and costly)
- Tubes are filled with cement or grout to prevent steel corrosion
Asphalt
Consists of a hard aggregate (basalt) and bitumen as the matrix. Asphalt is laid hot and when it cools, the bitumen solidifies.
The material exhibits greater toughness than concrete and thus can deal with slight movements in the road better
Laminates
Composites that consist of varying materials sandwiched together
- Plywood is a laminate that consists of layers of timber with the grain arranged at 90° to each successive layer (removes directional weaknesses of the grain)
- Laminated veneer lumber (LVL) is similar to plywood but layers all in same direction (stronger than an equivalent piece of timber and less susceptible to shrinking or warping)
- LVL cross sectional size is not limited by tree size and defects do not pass through the entire beam
Geotextiles
Woven polymers or ceramic fibres that are often used to stabilise road bases, preventing potholes and can also used as filters for drainage systems
- Separates, filters, reinforces, protects and drains soil under the road surface
Dry Corrosion
Occurs through chemical reactions of metals or alloys with gases usually at high temperatures (usually reaction with oxygen)
Wet Corrosion
Occurs when a metal is placed into a fluid or electrolyte, where anode loses material and deteriorates and the cathode gains a layer of rust
Uniform Attack (Wet)
If a metal is placed in an electrolyte, some parts will become anodic and other cathodic but the locations of the cathode and anode continually change and thus uniformly corrodes
Galvanic Corrosion (Wet)
Occurs when dissimilar metals are placed together in a corrosive environment. Different metals have affinity to corrode, one metal becomes cathodic and the other anodic
- Can also occur microstructurally, for example anodic ferrite and cathodic cementite in steels
- On a reactivity table showing the extent that metals are anodic or cathodic compared to one another, the closer the two metals are, the slower the rate of corrosion
Concentration Cells (Wet)
Occur when there is a difference in concentration of an electrolyte
- In a metal container open at the top with water, the water at the top will have a higher concentration of O2 compared to the bottom, creating a concentration differential
- The high O2 area will create a cathodic area while the area with low O2 will become anodic
- Crevices can form concentration cells and cause crevice corrosion
Stress Cells
Areas of high stress tend to become anodic whereas areas of low stress tend to become cathodic
Stress cells can also occur microstructurally, as grain boundaries tend to be a location of high stress.
Metal alloys with a fine grains are more liable to corrode internally than a coarse grain metal
Passivity
For some metals, such as aluminium, surfaces corrode and form a non-porous protective layer preventing further corrosion (rust in steel is porous/flaky and allows for further corrosion)
- These metals exhibit a property called passivity
Protecting Against Corrosion
Hot-dip galvanisation - Zinc layer on steel and protects it from corrosion - sacrificial layer because anodic
the heat will severely distort the shape on thinner metal objects - thus galvanisation is done through electrolytic - zinc is deposited onto the sheet metal from an electrolyte in solution by electroplating
An alternative method involves the coating with zinc-rich paint
- Aluminium alloys can be anodised to create thicker oxide layers to further protect against corrosion
- Sacrificial anodes are blocks made of a more anodic material which are bolted to an object and corrodes instead of the structure (more effective when submerged in an electrolyte)
- Impressed current cathodic protection (ICCP) systems use a current to reverse the standard electrical current associated with corrosion and make the object cathodic - used in pipelines and long structures where sacrificial anodes would be ineffective over a long distance
- When riveting sheet metal, rivets should be the same metal or have similar reactivity to prevent dissimilar metal corrosion (e.g. stainless steel rivets should not be used on sheet aluminium)
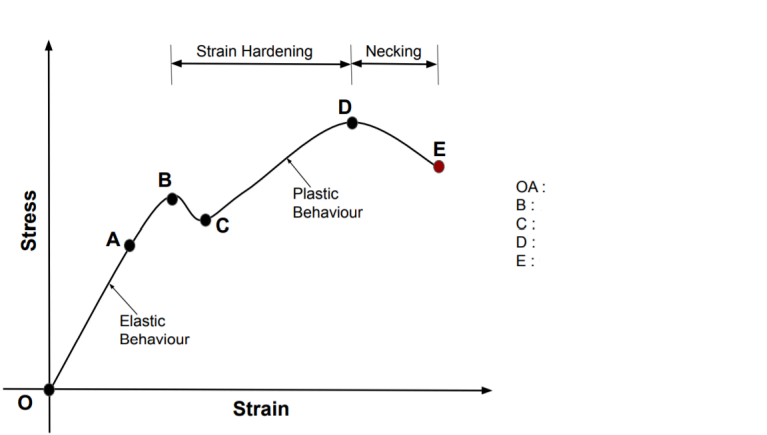
name B C D E OA
