Periodic Table Chemistry Test
1/37
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
38 Terms
Periodic Table
Tool for organizing the elements
Periods
Rows on the periodic table
Groups
Columns on the periodic table
Periodicity
Periodic Table patterns and trends on the periodic table
Atomic Number
Number of protons in an atom
Atomic Mass
Mass in grams of 1 mole of atoms (6.02 x 10^23)
Atomic Radius
Half the distance between two nuclei of the same element when bonded together
Electronegativity
Ability of an atom, in a chemical bond, to attract the shared valence electron to itself
Ionization Energy
Energy required to remove the outermost electron from an atom
Ion
Atom with a charge
Cation
Positively charged ion. Results from loss of electrons
Anion
Negatively charged ion. Results from gain of electrons
Alkali Metals
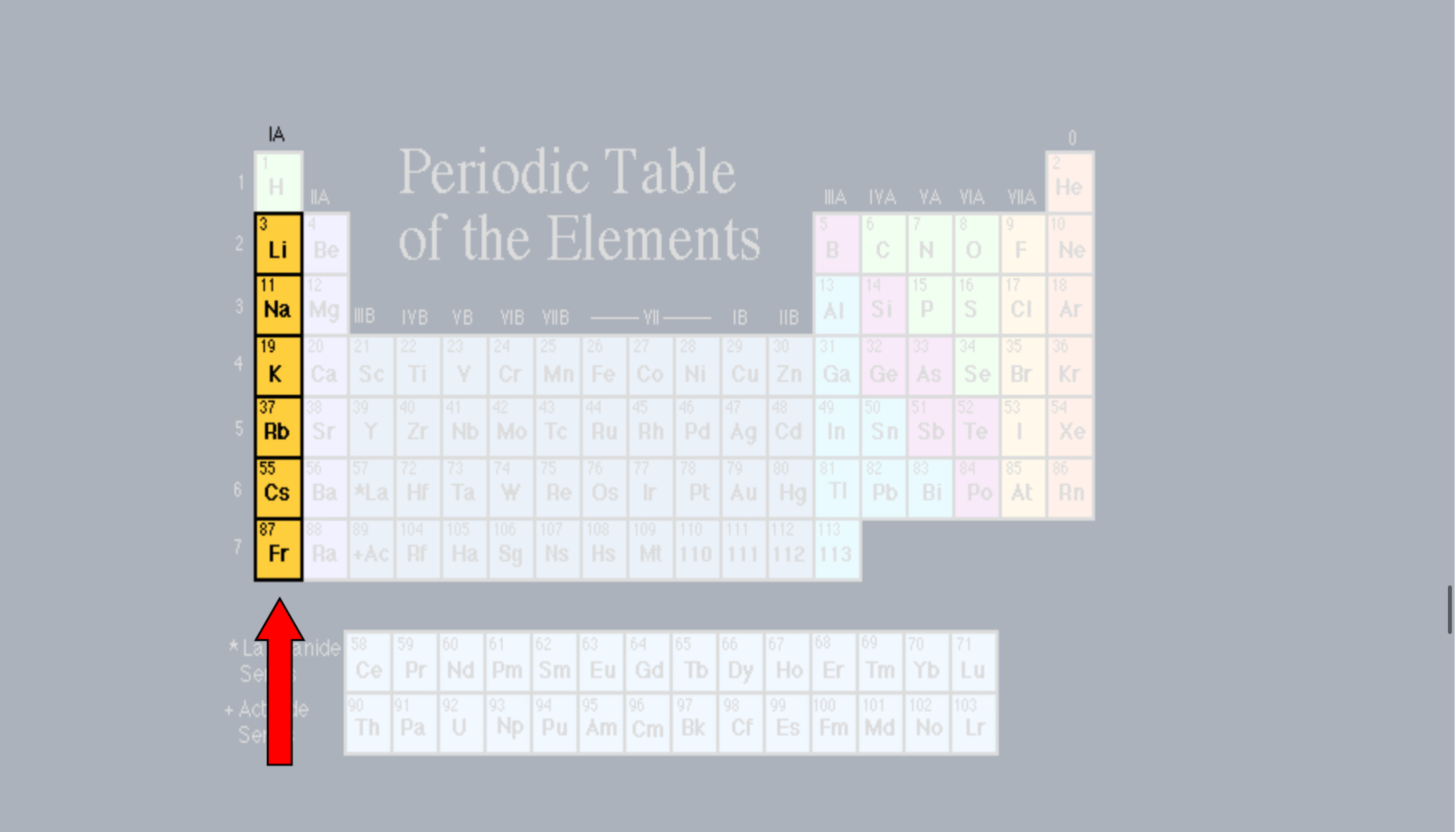
Alkaline Earth Metals
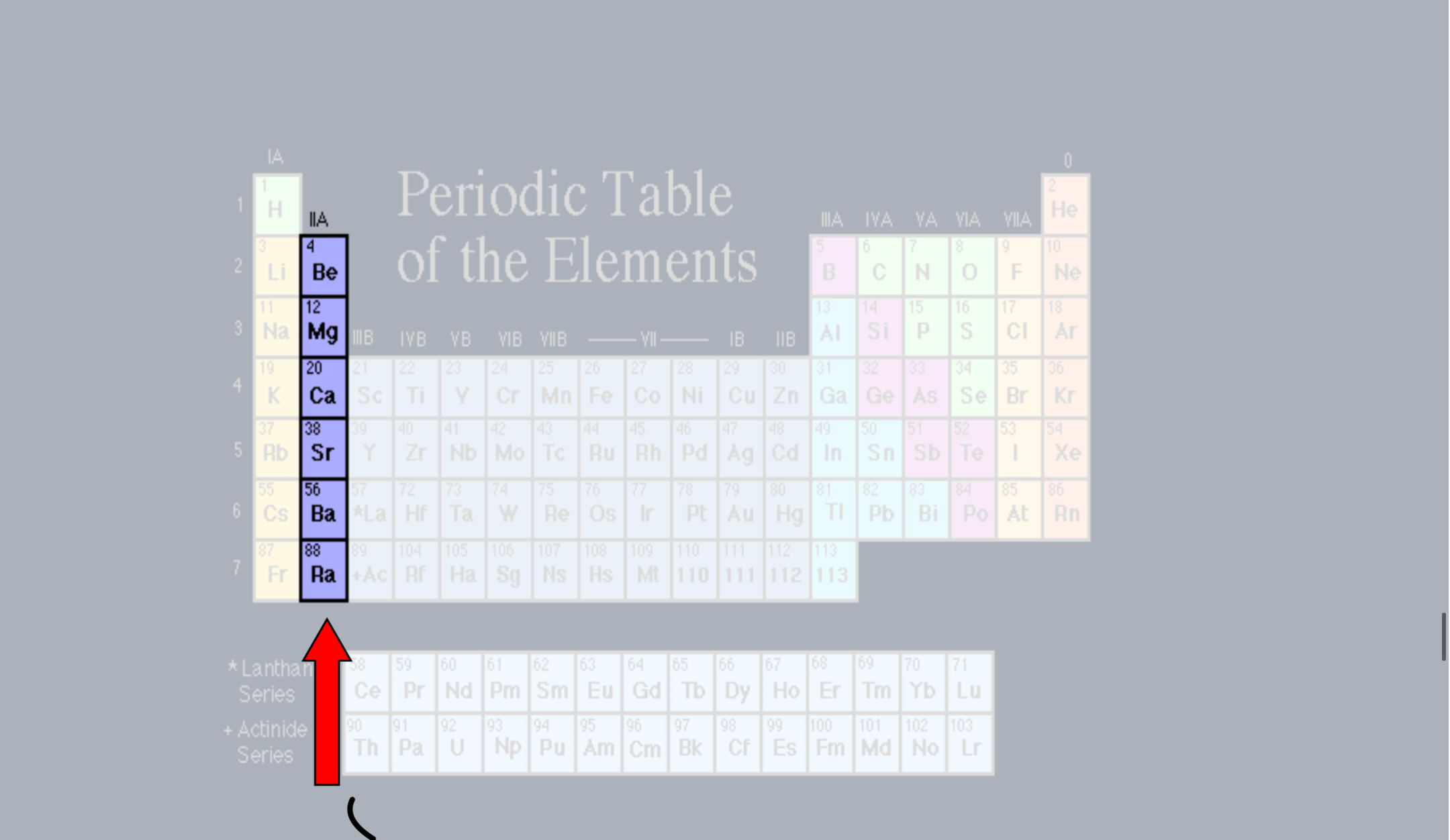
Transition Metals
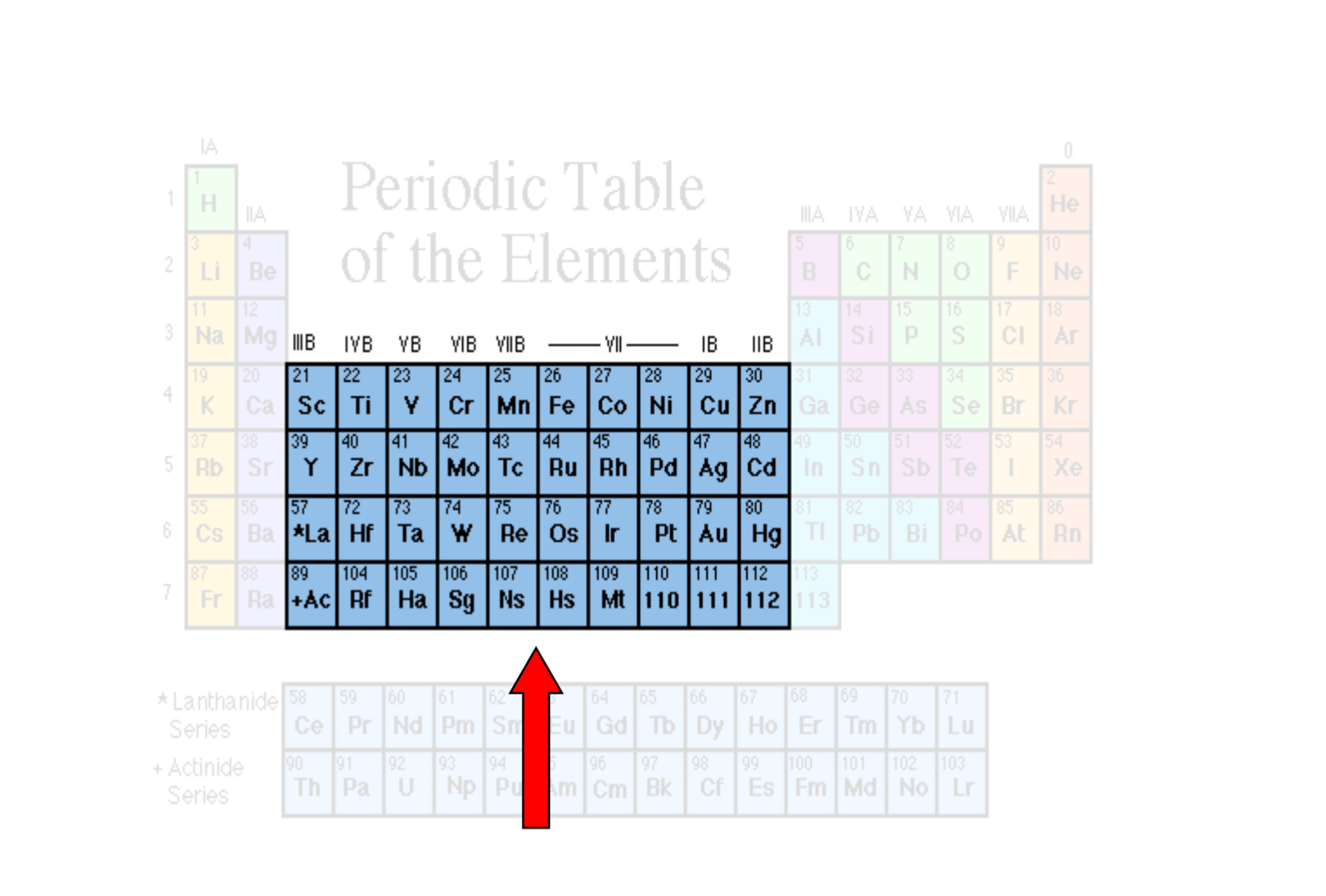
Halogens
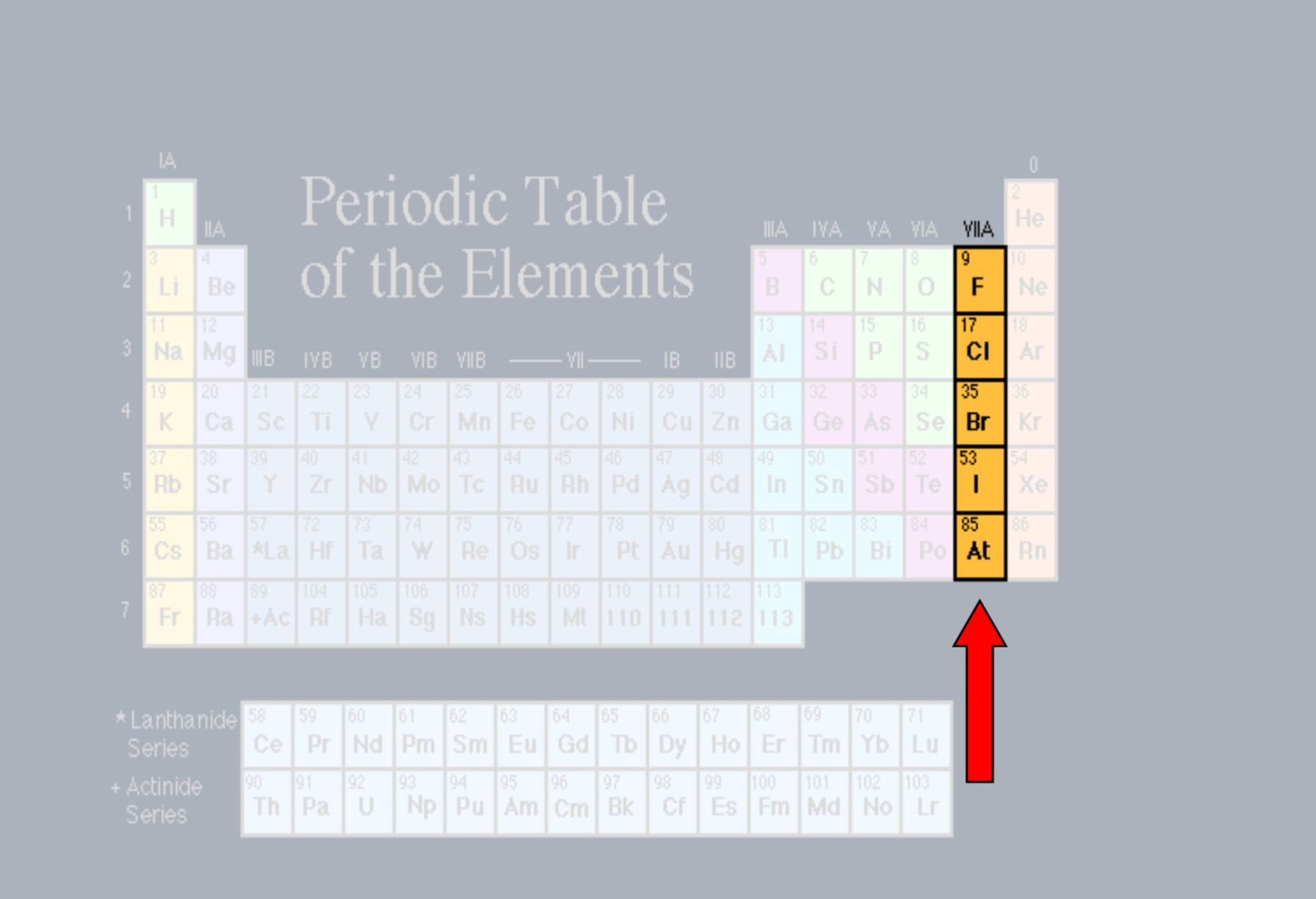
Nobel Gases
Lanthanides
Actinides
Representative Elements
Smaller
Cations have __ radii than their parent atom
Larger
Anions have __ radii than their parent atom
+1
Each time an electron is lost in an element a charge of __ is added
Increase
As radii decreases, electronegativity, ionization energy and electron affinity __
Decrease
As radii increases, electronegativity, ionization energy and electron affinity __
Decrease
As charge is added to an element, electrons and radii
Johann Dobereiner
In 1829, he classified some elements into groups of three, which he called triads. Elements in triads had similar chemical and orderly physical properties.
John Newlands
In 1863 suggested "octaves" because after being in order of increasing atomic mass certain properties repeated every 8th element.
Dmitri Mendeleev
1869 he published a table of elements organized by increasing atomic mass and left vacant spaces were unknown elements should fit
Lothar Meyer
1869 published a table with elements organized by increasing atomic mass and with spaces for unknown elements, but doesn’t get the credit
Henry Moseley
1913 determined atomic number of the elements and rearranged based on that; died in WWI
Glenn T. Seaborg
Co-discovered 10 new elements; moved 14 elements to below the lanthanide series in 1944; only person to have an elements named after him while still alive
Metallic Character, Atomic Radius, Ionic Radius,
Across: decreases
Down: Increases
Ionization Energy, Electronegativity
Across: Increases
Down: Decreases
Ionic Radius
Measure of the relative size of ions
1
Valence Electrons in Alkali Metals
2
Valence Electrons in Alkaline Earth Metals, Transition Metals, and Actinides/Lanthanides
3-8
Valence Electrons going across from the group boron is in to the noble gasses