CHM 244 - nucleophile strength (formulas)
1/40
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
41 Terms
H2S
hydrogen sulfide
pka = 7
moderate
R2S
moderate
R-SH
thiol
pka = 10-12
moderate
NH3
ammonia
pka = 32-38
moderate
RNH2
moderate
R2NH
moderate
R3N
amine
moderate
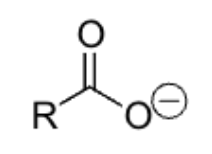
moderate
stronger nucleophiles have a __________ charge
negative
SR-
strong
SH-
strong
CN-
cyanide
strong
OR-
strong
OH-
hydroxide
strong
NH2-
strong
NHR-
strong
NR2-
strong
CH3-
strong
CH2CH3-
ethane
strong
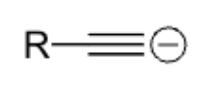
strong
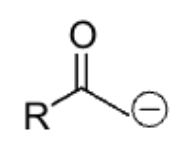
strong
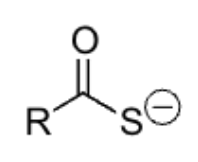
strong
I-
strong nucleophile
H2O
pka = 15.7
weak
R-OH
alcohol
pka = 16-18
weak
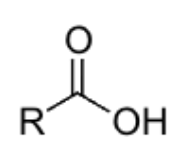
carboxylic acid
pka = 4-6
weak
Cl-
weak
Br-
weak
F-
weak
HSO4-
hydrogen sulfate ion
very weak
SO42-
sulfate
very weak
NO3-
nitrate
very weak
ClO4-
very weak
H2PO4-
dihyrdogen phosphate
pka = 7.21
very weak
HPO42-
hydrogen phosphate
pka = 12.3
very weak
PO43-
phosphate
very weak
NH4+
ammonium
pka = 9.2
non-nucleophilic
lewis acids
non-nucleophilic
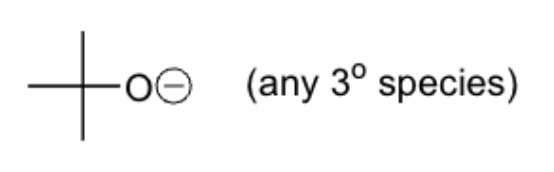
non-nucleophilic

non-nucleophilic
cations
Ex. Na+, Ca2+
non-nucleophilic