Module 3: Principles of Gram-Positive Bacteria with other Gram-Negative Bacteria and Antimicrobials
1/328
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
329 Terms
What bacteria are catalase positive?
• Staphylococcus spp. (used to different from Strep which is negative)
• Listeria
• H. Pylori
What bacteria are coagulase positive?
• Staphylcoccus aureus (used to differentiate between other Staph species)
What bacteria do you have to worry about being a contaminant?
• Staph epidermis
• B Cereus
• Species of Corynebacterium that are not diphteriae
What bacteria are beta-hemolytic?
• Staph aureus
• Strep pyogenes
• Strep agalactiae
• B. cereus
What bacteria are alpha-hemolytic?
• Strep pneumoniae (sensitive for Optochin test)
• Viridians streptococci (resistant for Optochin test)
What bacteria are gamma-hemolytic?
• Streptococcus bovis/gallolyticus
• Enterococcus faecalis & faecium
• B. anthracis
What is sensitive to the Optochin test?
• Strep pneumoniae
What has no Lancefield group?
• Strep pneumoniae
• Viridians streptococci
What is in Lancefield Group A?
• Streptococus pyrogenes
What is in Lancefield Group B?
• Streptococcus agalactiae
What is in Lancefield Group D?
• Streptococcus bovis/gallolyticus
• Enterococcus faecalis & faecium
What bacteria has a capsule?
• Streptococus pyrogenes
• Streptococcus pneumoniae
• Streptococcus agalactiae
• Bacillus species
• Haemophilus spp.
• Bordetella pertussis
• Neisseria meningitidis
• Bacteroides fragilis
Staphylococcus aureus
• Golden pigment
• Gram positive cocci in clusters
• Beta-hemolytic
• Catalase: Positive
• Coagulase: Positive
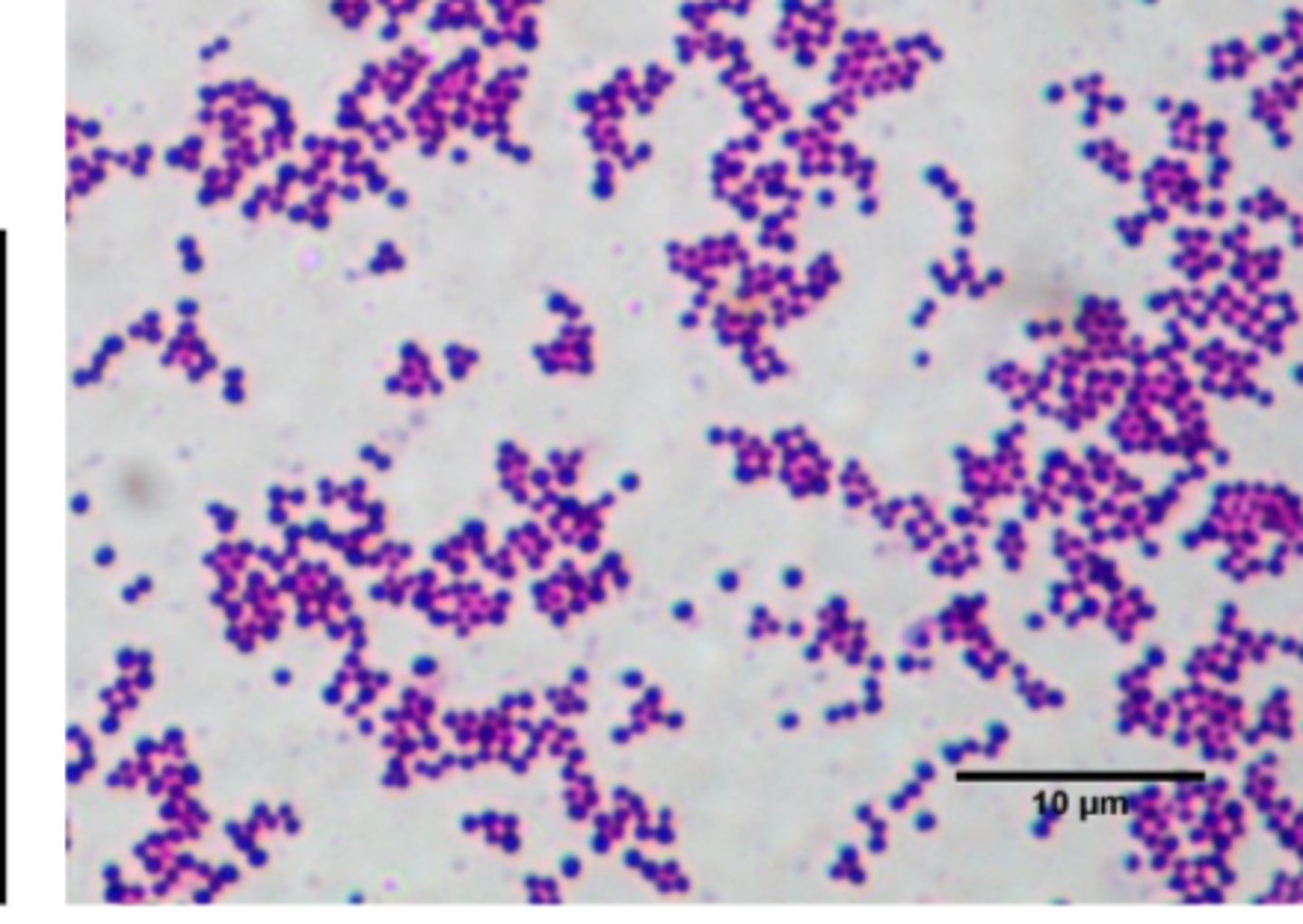
What test is used to differentiate between Staph and Strep?
• Catalase test
• Positive in Staph and negative in Strep
• Measures bacteria's ability to use catalyze hydrogen peroxide into water and oxygen (which appears as gas bubbles)
What are the coagulase results for staphylococci?
– Coagulase positive - S. aureus
– Coagulase negative- S. epidermidis, S.saprophyticus
Which staph stains are coagulase negative?
S. epidermidis, S. saprophyticus
Pathogenesis of S. aureus
• Exotoxin release
- 1) Gastroenteritis
> N/V, diarrhea, abdominal pain
> Comes from food
- 2) Staphylococcal scalded skin syndrome
> Affects neonates w/ local local infection of the umbilicus or older children with skin infections
> Cleavage of middle epidermis
> Healing is rapid with low mortality
- 3) Toxic Shock Syndrome
> TSS Toxin-1 (TSST-1)
> Associated with wound infections, tampon-use in menstruating women, childbirth, and abortions
> Potent stimulator of both tumor necrosis factor (TNF), interleukin-1, and interleukin-2
> Sudden onset of high fever, nausea, vomiting, and watery diarrhea
> Diffuse erythematous rash- palms and soles undergo desquamation
> Septic shock- hypotension and severe organ system damage
• Diseases resulting from direct organ invasion
- Pneumonia
– Meningitis
– Osteomyelitis
– Endocarditis
– Septic arthritis
– Skin infections
Which antibiotics treat endotoxins directly?
None, they are not effective and Ig therapy may be needed for these symptoms.
Resistance Mechanisms in S.aureus
1) Penicillinase production
2) MecA gene - alteration in PBP2 → PBP2A
> DOC: vancomycin, daptomycin, linezolid
3) Cell wall thickening- and prevent vanco from entering the cell; Vancomycin Intermediate S. aureus; overproduction of D-Ala D-Ala (VISA)
4) vanA gene- Vancomycin Resistant S. aureWhat us (VRSA)
> D-Ala D-Ala → D-Ala D-lac
vanA
• A gene that S. aureus expresses D-Ala D-lac rather than D-Ala D-Ala so vancomycin is unable to bind
• No bueno, the organism is classified as VRSA
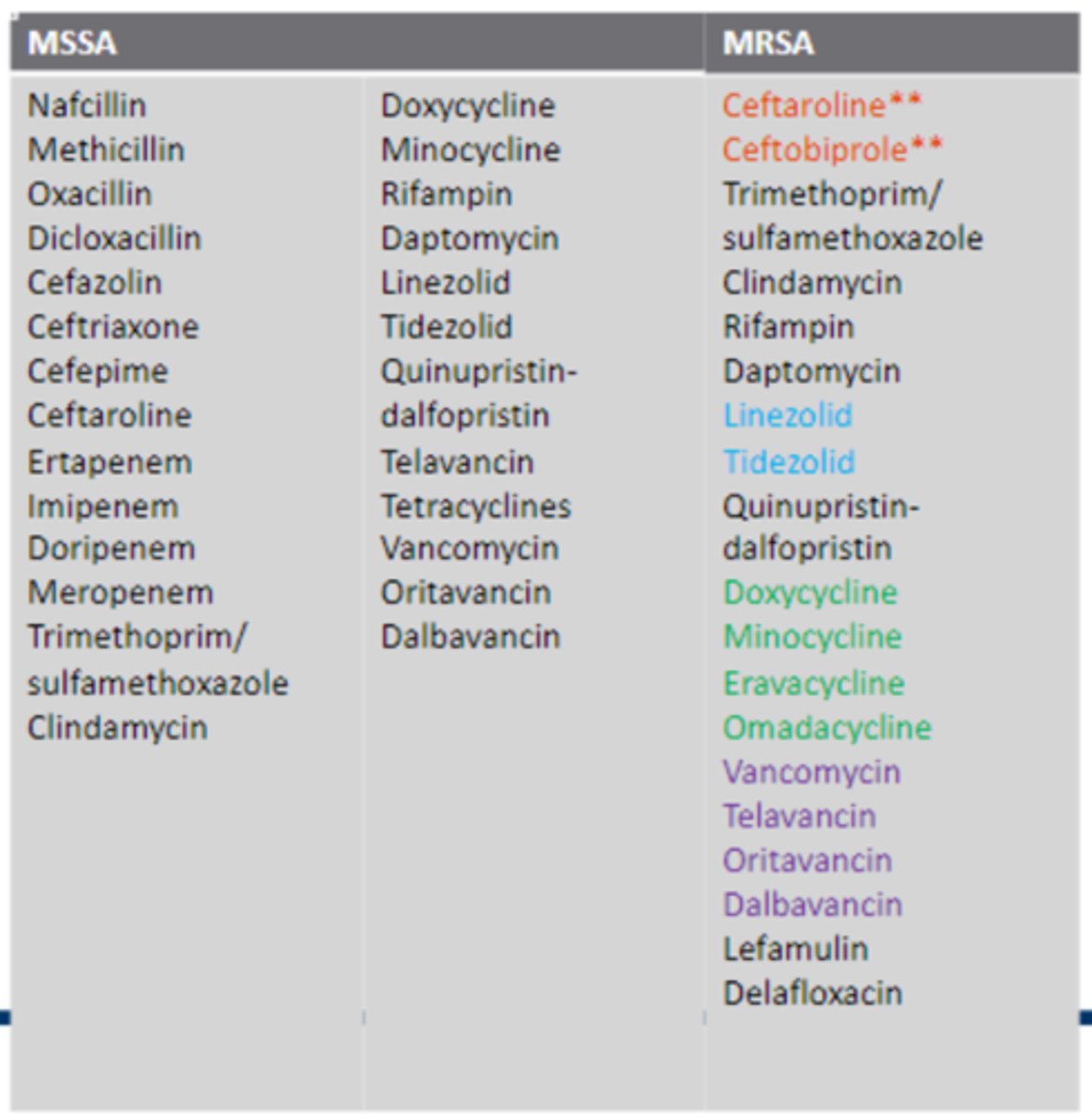
What is MRSA?
• The bacteria has the mecA gene
• They are resistant to the antistaphycoccal penicillins like oxacillin, methicillin, etc.
What drugs are used to treat MRSA?
• Vancomycin
• Daptomycin
• Lipoglycopeptides. (Telavancin, Dalbavancin, Oritavancin)
• Linezolid, Tedizolid
• Tetracyclines
• Clindamycin (after doing the D test)
• Ceftaroline
• Ceftabiprole
• Dexafloxacin
• Bactrim
- Can be given orally
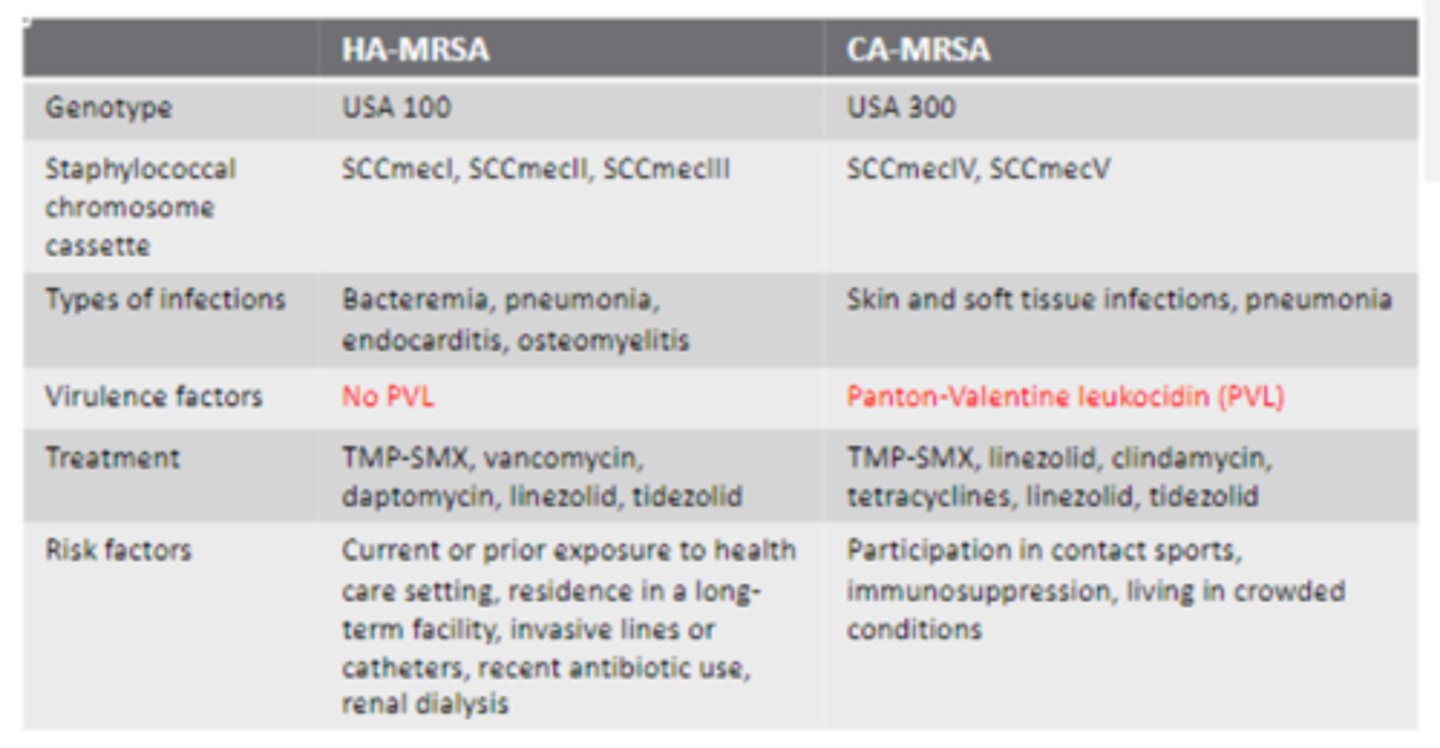
Healthcare-associated vs. Community- associated MRSA
• More deep-seated infections for HA-MRSA (Bacteremia, pneumonia, endocarditis, osteomyelitis) compared to SSTI and pneumonia for CA
• Panton-Valentine leukocidin (PVL) in CA-MRSA and NOT HA-MRSA
> Exotoxin that causes a lot of tissue destruction (nectrozing pneumonia and SS tissue infect.)
• Similar treatment
Treatment of S.aureus
(See Image)
CDC core prevention strategies
– Assessing hand hygiene practices
– Implementing contact precautions
– Recognizing previously colonized patients
– Rapidly reporting MRSA results
– Providing education to clinicians
– Universal decolonization
Is universal or targeted decolonization more effective for MRSA?
Universal decolonization using IN mupirocin and chlorhexidine baths
S. epidermidis
• Gram stain: positive
• Catalase: positive
• Coagulase: negative
• Contaminant in blood cultures
- Normal skin flora
• Commonly associated with prosthetic device and intravascular catheters
– Polysaccharide capsule
– Biofilms
S. saprophyticus
• Gram stain: positive
• Catalase: positive
• Coagulase: negative
• Leading cause of urinary tract infections
• Commonly acquired by females in the community
Streptococcus
• Facultative anaerobe
• Gram stain: positive
• Chains
• Catalase: negative
Blood agar hemolysis Classification
– Alpha (α) hemolytic: partial
– Beta (β) hemolytic: complete
– Gamma (ɣ) hemolytic: no hemolysis
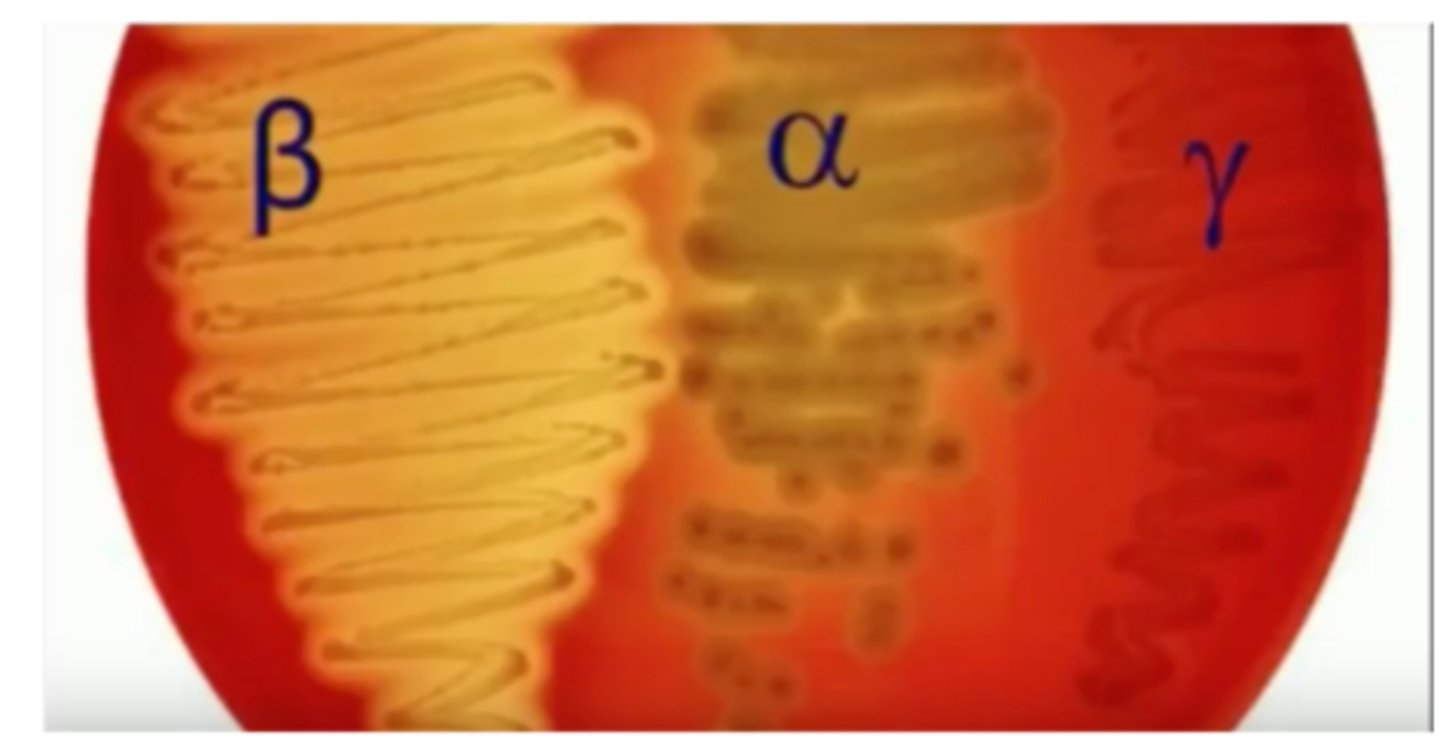
Cell wall antigen by Lancefield
– Serology: antibody-antigen of carbohydrates on surface
– Groups A through S
• Streptococcus pneumoniae is sensitive and Viridians streptococcus is reistant
Optochin test
Assist in differentiating alpha hemolytic streptococci
– Drug/chemical
– Results: Sensitive or Resistant
– Very selective according to the species of streptococc
Optochin test is used to differentiate between __________ hemolytic streptococci.
alpha
Results: Sensitive (Strep . pneumoniae) or Resistant (Viridians strep)
Streptococcus pyogenes
• Gram stain: positive
• Catalase test: negative
• Beta-hemolytic
• Lancefield Group A antigen
– Nickname: Group A streptococci (GAS)
• Commonly found – Normal Microbiota (Normal Flora)
– Skin (opportunistic)
• Virulence Factors
- Hyaluronic acid capsule
- M protein
- Cell wall (Proteins + Lipoteichoic acid (LTA)
- Enzymes
- Toxins
• Enzymes: Streptokinase, Deoxyribonucleases, Hyaluronidase
• Toxins: Pyrogenic exotoxins, Hemolysins
Capsule and Cell Wall of S. pyogenes
• Enveloped in a hyaluronic acid capsule
– Retards phagocytosis from polymorphonuclear leukocytes and macrophages
–Molecularly similar to human connective tissue
– Bind to epithelial cells
– Disruption of intercellular junction
• M protein
– Filamentous macromolecule
– Alpha helical coiled coil structure
– Binding to host proteins: Adherence to human cells
- Inhibits the alternate complement system
Extracellular Products: Enzymes of S. pyogenes
• Streptokinase: activates plasmin
– Digest fibrin → to escape from blood clots
• Deoxyribonucleases: degrade DNA
– Facilitate the spread in tissue by liquefying pus
• Hyaluronidase: splitting hyaluronic acid
– Cleaves hyaluronic acid in human connective tissues
– Allows the bacteria to spread
• Allow of the enzymes makes it easier for the bacteria to spread
Extracellular Products: Toxins of S. pyogenes
• Pyrogenic exotoxins (Spe): A, B, and C
– Superantigens – stimulating T cells
– Release cytokines that mediate shock and tissue injury
• Hemolysins (streptolysin O and S)
– O: lyses erythrocytes (RBCs), leukocytes, and platelets
– S: lyses erythromycin (the medication??), leukocytes, and platelets
• Stimulates the release of lysosomal contents AFTER engulfment → killing phagocyte
Pathogenesis of of S. pyogenes
• Humans are natural reservoir
• Oropharynx: 5-15%–
No sign and symptoms of disease
• Port of entry determines the infection
– Tissues with direct contact with infected
• Every case: diffuses rapidly
– i.e. tissue → lymphatics → bloodstream
- Explains the sudden onset
Infectious Diseases Attributable: S. pyogenes
• Skin and soft tissue infections
– Erysipelas - infect. outer skin layers
– Cellulitis
– Necrotizing fasciitis (gangrene)
– Impetigo (pyoderma)
• Pharyngitis (strep throat)
• Puerperal fever (maternal death)
• Scarlet fever
• Toxic shock syndrome
• Delayed (poststreptococcal; when left untreated)
– Glomerulonephritis
> 1 week after skin or pharyngitis
– Rheumatic fever
> Most serious
> 1-5 weeks after pharyngitis
Treatment options for S. pyogenes
• Penicillin – DOC
• Amoxicillin – DOC
• Erythromycin* (probably due to streptolysin S which lyses erythromycin)
• Azithromycin
• Clarithromycin
• Clindamycin
• Cefazolin
• Cephalexin
**Think back to skills lab
***Same DOCs as S. agalactiae***
Which of the following infections is related to GAS?
Pharyngitis
Streptococcus pneumoniae
• Catalase test: negative
• Alpha-hemolytic
• Lancefield Group: none–
Lacks the carbohydrate antigen
• Optochin test: sensitive (susceptible)
• Sometimes identified as diplococci or short chains
• Commonly found – Normal Microbiota (Normal Flora)
– Upper respiratory tract (5-40%)
• Virulence Factors
- Capsule polysaccharide
- Cell wall (Proteins + Teichoic acid (TA) + peptidoglycan)
- Surface protein A and C
- Enzymes - Pneumolysin
- Resistance - changes to PBP proteins
Capsule and Cell Wall of S. pneumoniae
- Capsule polysaccharide can cause disruption in the intracellular junctions to allow the microorganisms to remain EC as they penetrate around the epithelium of tissues and allows it to evade phagocytosis
- Cell wall is the peptidoglycan and TA and it allows it to evade phagocytosis, evade the complement system and prevent mechanical clearance by mucous secretion
Extracellular Products: Proteins of S. pneumoniae
• Surface protein A
– Inhibits complement pathway (C3b to factor B)
– Allows binding to epithelial membranes
• Surface protein C (choline binding protein A)
– Inhibits complement pathway (factor H)
– Inhibits transportation of antibodies across the epithelial cells
> Inhibiting polymeric immunoglobulin receptor
Extracellular Products: Enzyme of S. pneumoniae
• Pneumolysin (exotoxin)
– Produced by invasive strains
– Released during autolysis –lytic to host cells
– Inhibit ciliary action of epithelial cells
– Impairs respiratory burst of phagocytic cells (oxidative burst)
–Active inflammation
> Induce production of chemokines and cytokines
Antibiotic Resistance of S. pneumoniae
• Penicillin-resistant (1967)
• Alteration to the penicillin-binding protein (PBP)
– Reducing affinity
– PBP2b
> Lower affinity to penicillin
– PBP2x
> Lower affinity to cefotaxime
**Think back to skills lab**
Pathogenesis of S. pneumoniae
• Extracellular bacterial pathogen
• Adhere to mammalian cell
– Replicate and failed to be cleared
• Causes disease due to ability to produce an intense inflammatory response (pneumolysin; mortality can be high)
• In most organs: lungs, middle ear, central nervous system
Infectious Diseases Attributable to S. pneumoniae
• Pneumonia
• Sinusitis
• Bronchitis disease (classic infection)
• Otitis media
• Meningitis
• Bacteremia
• Invasive pneumococcal disease (classic infection)
- Respiratory → Blood → CNS (Meningitis)
Predispose to Pneumococcal Infection
• COPD
• Asplenia
• Asthma
• Alcoholism
• Smoking
• Cirrhosis
• Diabetes mellitus
• Renal insufficiency
• Treated with glucocorticosteroids
• Shelters for the homeless
• Daycare centers
• Military training camps
• Prisons
Treatment Options of S. pneumoniae
• Penicillin*
• Amoxicillin *
• Azithromycin*
• Clarithromycin*
• Clindamycin
• Ceftriaxone
• Cefotaxime
• Cefdinir
• Cefpodoxime
• Vancomycin
• Levofloxacin
• Moxifloxacin
• Best option is prevention
- Vaccination
• PPSV23
* = resistance

Streptococcus agalactiae
• Catalase: negative
• beta-hemolytic
• Lancefield Group B antigen
– Nickname: Group B streptococci (GBS)
• Commonly found – Normal Microbiota (Normal Flora)
– Lower gastrointestinal tract (5-30%)
– Vagina
• Virulence Factors
- Capsule polysaccharide evades phagocytosis and inhibits the complement pathway
- Surface proteins: adherence and evasion
- Induce CK release (TNF-α, IL-8, IL-1β, and IL-6)
> Cause inflammation
> Damage tissues
Pathogenesis of Streptococcus agalactiae
• Extracellular bacterial pathogen
• Colonize mucosal surface then breach surfaces to enter normally sterile sites e.g. blood stream
• Adhere to the vaginal epithelium, placental membrane, and respiratory tract epithelium
• Cross the epithelial barrier by paracellular route and translocation of the epithelial barriers for dissemination
– Pilus surface protein
What has pili?
• S. galactiae
• Enterococcus
• Haemophilus influenzae
• Legionella
• Neisseria
Infectious Diseases Attributable to S. agalactiae
• Pregnancy related (since it is found in the vagina):
– Bacteremia
– Sepsis in neonates
– Meningitis in neonates
• Non-pregnancy related:
– Bacteremia (female or male)
– Female genital tract infection
– Skin and soft tissue infection
– Osteomyelitis
– Arthritis – knee, shoulder, hip joints
Treatment Options of S. agalactiae
• Penicillin – DOC
• Amoxicillin – DOC
• Clindamycin*
• Vancomycin
***Same DOCs as S. pyogenes***
Viridans streptococci
• Catalase: negative
• Alpha-hemolytic*
• Optochin test: resistant
• Lancefield Group: None
• Commonly found – Normal Microbiota
– Upper respiratory tract
– Female genital tract-
– Gastrointestinal tract (all) including oral cavity - causes dental caries, intrabdominal infections, and endocarditis
• Group of streptococci
– Streptococcus mitus
– Streptococcus salivarius
– Streptococcus mutans*
Infectious Diseases Attributable to viridans streptococci
• Endocarditis
• Intra-abdominal infections
• Dental caries (supragingival plaques)
(Just like Strep bovis except the dental caries is added)
Treatment options for Viridans streptococci
• Penicillin* – DOC
• Ceftriaxone
• Vancomycin
(Just like Strep bovis)
*rare to see resistance
Streptococcus gallolyticus/Streptococcus bovis
• Catalase: negative
• Gamma-hemolytic
• Lancefield Group D antigen
• Commonly found – Normal Microbiota (normal flora)
– Gastrointestinal tract
> Colorectal mucosa
Infectious Diseases Attributable to Streptococcus bovis
• Endocarditis
• Intra-abdominal infections
(Just like Viridians strep except it's missing dental caries)
Treatment options to Streptococcus bovis
• Penicillin* – DOC
• Ceftriaxone
•Vancomycin
(Just like viridians strep)
Enterococci faecalis & faecium
• Catalase: negative
• Gamma-hemolytic
• Lancefield Group D antigen
• Commonly found – Normal Microbiota (Normal Flora)
– Gastrointestinal tract
• Virulence Factors
- Antigenic cell surface (Aggregation substance, E. faecalis substance protein)
- Enzymes (Gelatinase and serine proteases_
- Toxins (Cytolysin-hemolysin)
- Resistance to antibiotics
Cell Surface: Enterococci faecalis & faecium
• Aggregation substance
– Increases adherence and internalization into eukaryotic cells
– Enhance adherence to serum and extracellular matrix proteins
• Fibronectin, fibrin, collagen type I
• E. faecalis surface protein (ESP)
– Adhesin in the formation of biofilms
• Pili: major role in biofilm formation
Enzymes: Enterococci faecalis & faecium
• Gelatinase and serine proteases
– Facilitation of invasion
• altering immunoglobulins and/or complement molecules
– Development of biofilm
• Regulate autolysis
• Release high-molecular weight extracellular DNA to help hide itself and allow s it to nest iteself
– Degradation of host connective tissues
• Exposing ligands for bacterial attachment
Toxins: Enterococci faecalis & faecium
• Cytolysin-hemolysin
– Bacterial toxin
– Lysing eukaryotic cells
- Used to lyse RBCs, WBCs, and platelets
Antibiotic Resistance of Enterococci
• Cephalosporin resistance: intrinsically
– All enterococci (decreased affinity to PBP5)
• Most are penicillin resistance
– Built tolerance to bactericidal killing
– E. faecalis: Ampicillin – DOC
• Vancomycin resistance– More with E. faecium
– Ampicillin resistant
– vanA gene expression
– vanB gene expression
• E. faecium is more resistant than E. faecalis
What is the DOC for E. faecalis?
Ampicillin
If E. faecalis is vancomycin resistant, can you use its drug of choice ampicillin?
No, since if it is vancomycin resistant, it is also ampicillin resistant
Infectious Diseases Attributable to Enterococcus
• Health care-associated infections
• Urinary tract infections
• Wounds infections
• Intra-abdominal infections
• Endocarditis
• Sometimes polymicrobial
–Intra-abdominal infections
– Wound infections
Treatment options: Enterococcus
• Ampicillin – DOC E. faecalis
• Gentamicin – combination
• VancomycinR
• Telavancin* - VRE vanB
• Linezolid
• Daptomycin
• Tigecycline
• Quinupristine-dalfopristin – E. faecium
- VRE options
What is the DOC for VRE vanB?
Telavancin
Bacillus
• Gram stain: positive
– Large bacilli (rods)
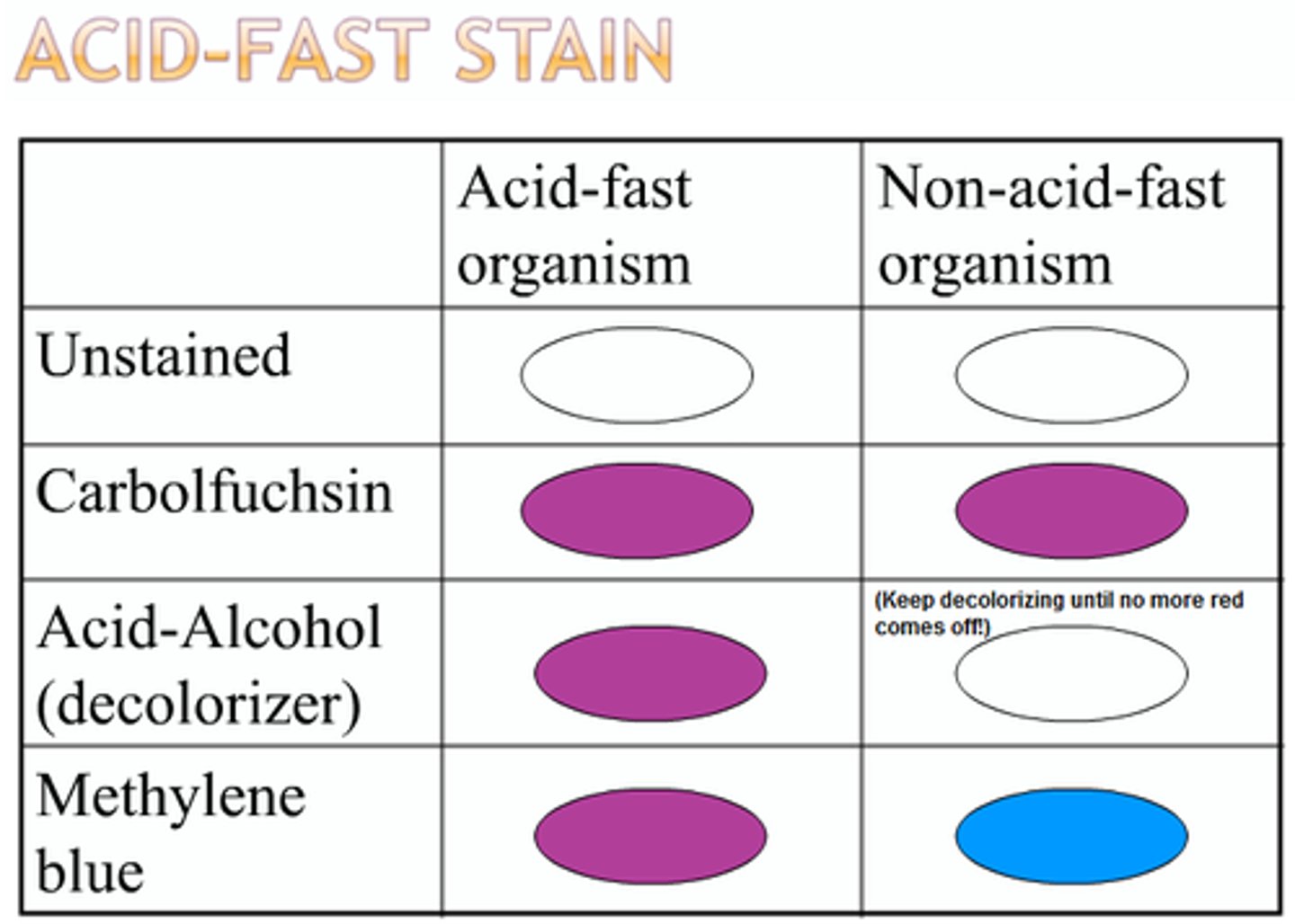
• Acid fast test: negative
– 1% hydrochloric acid • presence of mycolic acid in the cell walls(found in Mycobacterium)
• Facultative anaerobe
• Gray to white, ground glass colonies
– Comet trail or Medusa head
• Blood agar by hemolysis
– Beta (β) hemolytic: B. cereus
– Gamma (ɣ) hemolytic: B. anthracis
• Motility
– Non-motile: B. anthracis
– Motile: B. cereus (swarming)
• SPORE FORMING
• Ubiquitous - not associated with human infections
• Saphrophytic: needs only carbon and nitrogen • for energy andgrowth
What bacteria is saphrophytic?
Bacillus species
What bacteria are motile?
• B. Cereus
• H. Pylori
Acid Fast Test
1% hydrochloric acid - presence of mycolic acid in the cell walls (found in mycobacterium)
Virulence Factors: B. anthracis
• Toxins
– Protective antigen (PA)
– Edema factor (EF)
– Lethal factor (LF)
• PA allows to bind to a cell and forms a membrane channel
• PA & EF forms edema toxin to cause cell and tissue edema
• LF & PA forms lethal toxin to cause death for the host
– Impair both innate and adaptive immunity
– Allows proliferation of the bacteria
– Cell death in lungs which can cause hypoxemia

Virulence Factors: B. Cereus
• Toxins (depends on the food)
– More intoxication rather food borne illness
– Emetic (vomiting) toxin (cyclic peptide)
• Associated with spoiled rice, milk, and pasta
• Spores germinate and vegetative cells produce toxin during log phase
– Diarrheal toxin (enterotoxin)
• Sauces and meat dishes
• Spores develop to vegetative cells and secrete enterotoxin
– Induces fluid accumulation
– Other physiological responses in the small intestine
Infectious Diseases Attributable to Bacillus sp.
• B. anthracis
– (AKA anthrax)
– Cutaneous anthrax (~95%)
– Inhalation anthrax (~5%)
– Gastrointestinal anthrax (rare)
– Biological warfare/bioterrorism
• B. cereus
– Food poisoning
• Emetic type (1-5 hrs)
• Diarrheal type (1-24 hrs)
– Eye infections
– Contaminant in blood cultures
Treatment Options of Bacillus anthracis
• Ciprofloxacin OR Doxycycline PLUS combo of 1 - 2 agents below for severe, inhalation type
– Rifampin
– Vancomycin
– Penicillin or ampicillin
– Chloramphenicol
– Imipenem
– Clindamycin
– Clarithromycin

Treatment Options of Bacillus cereus
• No treatment for food poisoning
Corynebacterium diphtheriae
• Gram stain: positive
– Bacilli (rods) (sometimes club shaped/irregular)
• Acid fast test: negative– 1% hydrochloric ac›id • presence of mycolic acid in the cell walls (found inMycobacterium)
• Facultative anaerobe
• Non-sporulating & Non-motile
• Commonly found – Normal Microbiota
– Skin and mucous membrane (respiratory tract)
• C. diphtheriae– Most common species
– Unencapsulated
• Other species have been correlated to either contaminant of clinical specimens (blood cultures)
Virulence: C. diphtheriae
• Exotoxin (inhibits protein synthesis)
1. Requires presence of lysogenic β-phage
• Phage DNA integrates to bacteria
2. Absorbed into the mucous membranes
• Destruction of epithelium
• Superficial inflammatory response
3. Necrotic epithelium with exuding fibrin and red/white cells → grayish pseudomembrane that looks leatherly
• Tonsils, pharynx, and/or larynx
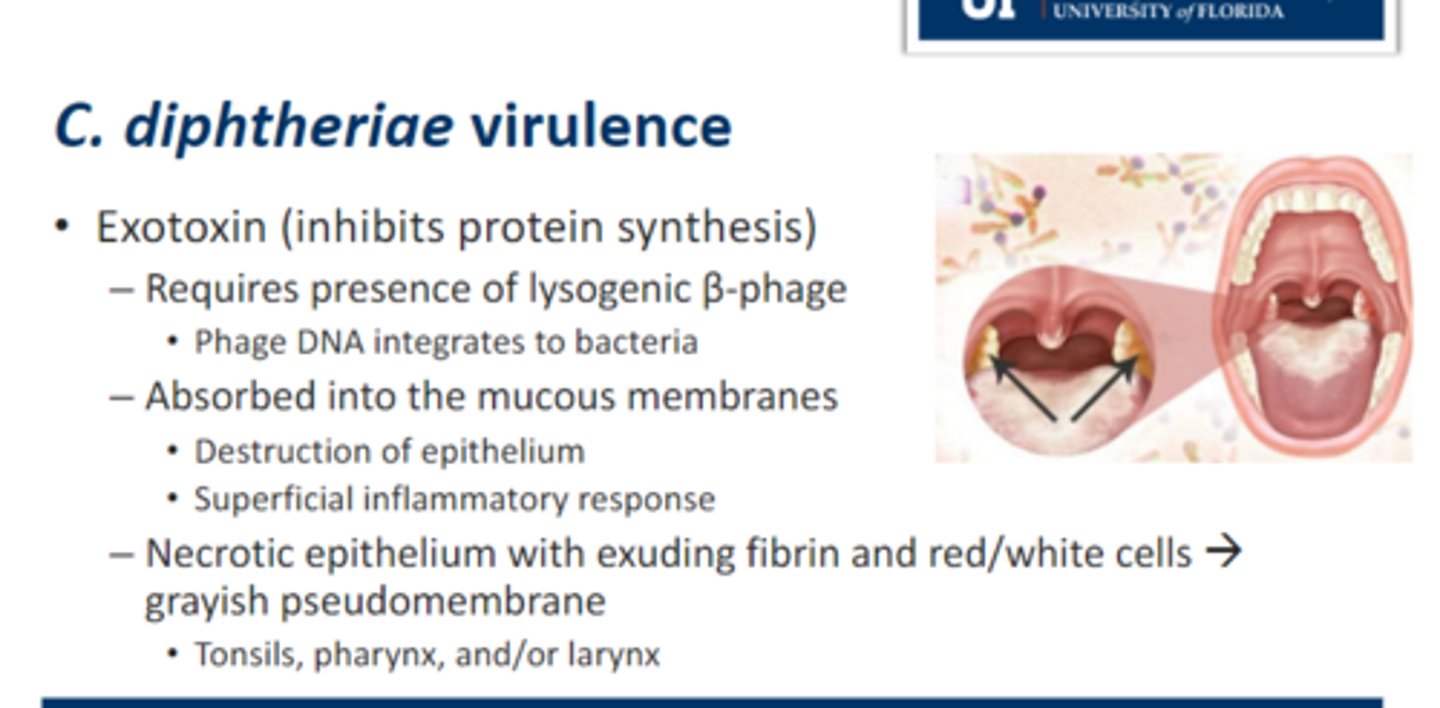
Infectious Diseases Attributable to C. diphtheriae
• Respiratory diphtheria
– Complications
• Myocarditis
– Heart failure
– Arrhythmias
– Death
• Neurotoxicity
– Neuropathy
• Cutaneous diphtheria
Treatment: C. diphtheriae
• Diphtheria antitoxin
– Neutralizes toxin before binding to tissue
• Antimicrobials
– Penicillin
– Erythromycin
• Vaccination
– Nontoxic immunogenic toxoid
– Given to children (DTaP)
– Booster every 10 years (Tdap)

Listeria
• Gram stain: positive
– Bacilli (rods) (short looking rods)
• Acid fast test: negative– 1% hydrochloric acid • presence of mycolic acid in the cell walls (found inMycobacterium)
• Facultative anaerobe
• Catalase test: positive
• Non-sporulating
• Commonly found
– Normal Microbiota
– Soil and water and some poultry and cattle.
– Present in raw milk
• Overcome food preservation and safety barriers (grows in canned foods)
- Grows at wide range of temperatures: 1-45°C (33.8-113°F)
- Low pH
- High concentration of salt ( Grows in canned foods)
• Virulence factors
- Adhesion proteins
- Surface proteins (internalins A & B): promotes phagocytosis and and then hides in it, and then produces listeriolysin O (ruptures the phagosome) which releases the bacteria and this process allows the bacteria to avoid antibodies
• Usually causes food borne illness (mild, self limiting febrile gastroenteritis), but it can escalate to meningitis or bacteremia in those that are immunosuppressed, elderly or neonates
Virulence: L. monocytogenes
• Enters the GI after ingestion of contaminated food
• Adhesin proteins
– Binds bacteria to the host cells
• Surface proteins - attracts macrophage and then hides in it (internalins A & B)
– Promotes phagocytosis
– Phagolysosome → low pH actives to produce listeriolysin O
• Releases bacteria
– Cycle continues and allows the bacteria to avoid antibodies
listeriolysin O
L. monocytogenes is able to infect phagocytes by dissolving the phagosome using this enzyme after using internalins A & B to be uptaken by macrophages
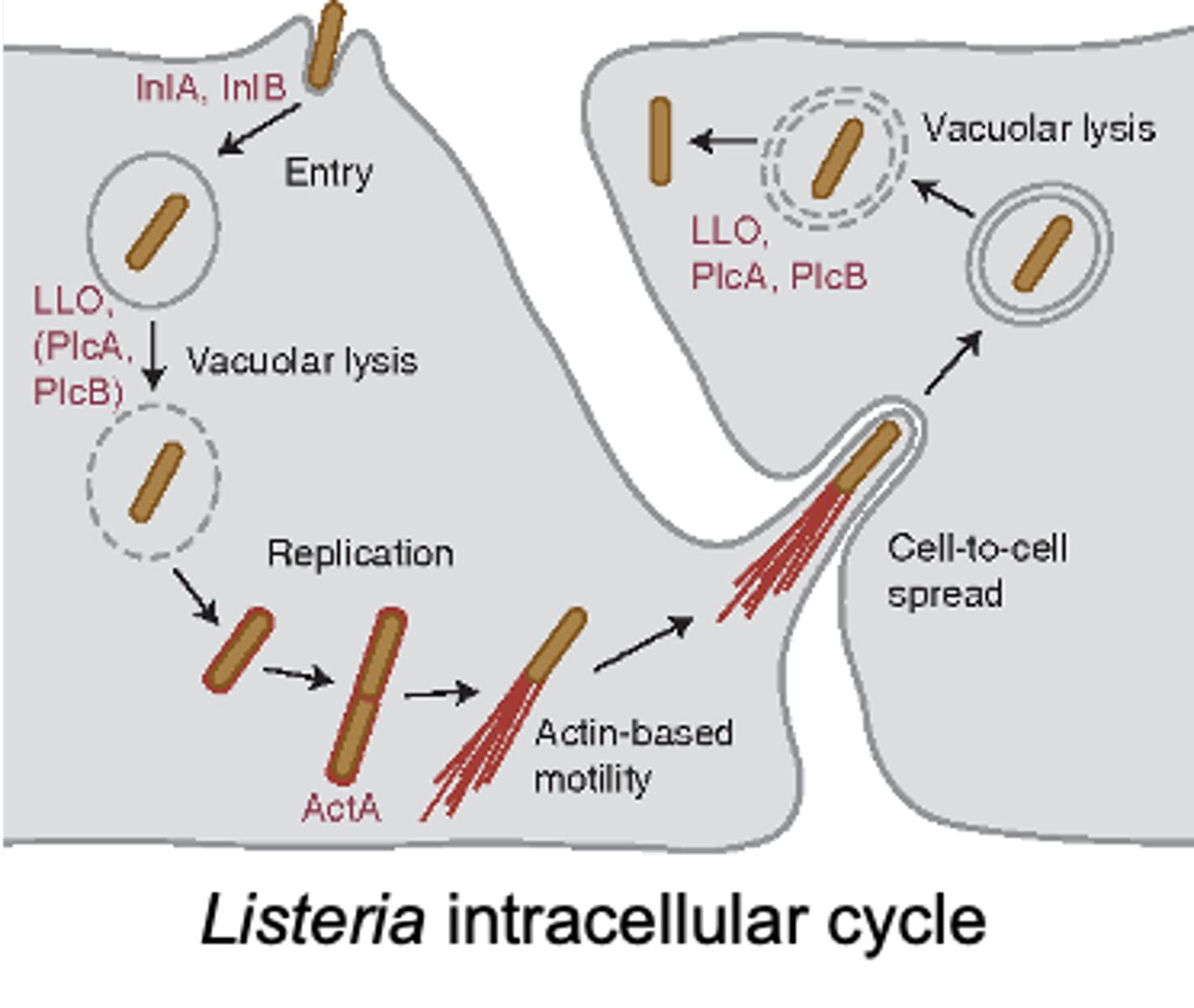
Infectious Diseases Attributable to L. monocytogenes
• Food borne illness
– Mild, self limiting febrile gastroenteritis for 1-3 days
• Immunocompromised
– Neonate, elderly, immunosuppressed
– Meningitis or bacteremia

Treatment: for L. monocytogenes
• Ampicillin
• Sulfamethoxazole-trimethoprim
Clostridium
• Gram-positive spore-forming rods
• Soil, decaying vegetation, intestinal tract
• Anaerobic
• Exotoxins and enzymes
• Four medically important species:
1. Clostridium botulinum
2. Clostridium tetani
3. Clostridium perfringens, 4. Clostridioides difficile v
Clostridium botulinum (Botulism)
• Lethal neurotoxin
(Botulinum neurotoxins (BoNTs)
• Blocks the release of acetylcholine (Ach) from presynaptic nerve terminals
– Flaccid muscle paralysis
• The fusion of AcH vesicles into the synaptic cleft is mediated by SNARE proteins, which C. botulism cleaves. So AcH cannot enter the synaptic cleft and the AcH cannot work foCr smooth muscle contraction.
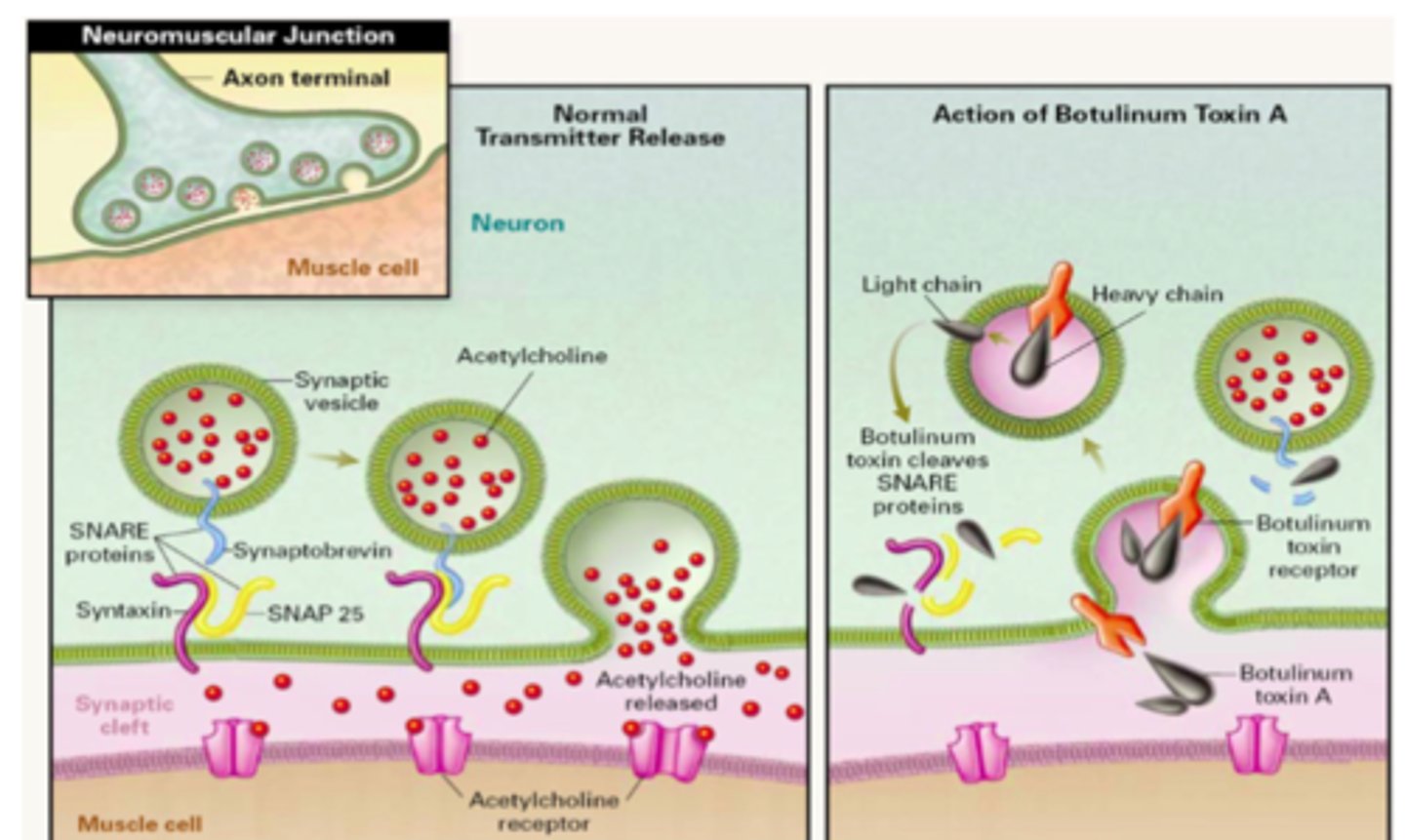
C. bolulism cleaves __________ proteins while C. tetani cleaves _________>
SNARE; synaptobrevin
Adult botulism
• Spores float in the air and can land on food
– Cooking food will kill spores
– Anaerobic environment from canning of smoking food
• C. botulinum will mature and synthesize neurotoxins
• Clinical manifestations: nausea, vomiting, diarrhea, abdominal cramps, diplopia, dysphagia, muscle weakness, respiratory paralysis, death
• Treatment with heptavalent (HBAT, Cangene Corporation) antitoxin prevents progression
– Only supplied from CDC
– Contains antibodies to 7 known botulinum neurotoxin serotypes (A-G)
– Equine serum
• Serum sickness

What bacteria forms spores?
• Bacillus spp.
• Clostridium spp.
Infant botulism
• Infants ingest food contaminated with C. botulinum
– Fresh honey contaminated with spores
– Spores germinate and colonize infant’s GI tract
• Clinical manifestations: constipation (2-3 days), muscle weakness “floppy babies”
• Treatment with human botulism immunoglobulin intravenous (BabyBIG®) that needs to be given ASAP
– Can be obtained from California Department of Public Health (Ok?? How useless)

Wound botulism
• Least common presentation of botulism
– Puncture wounds or deep space infections
– Spore from soil or environment germinate and produce toxin
• Similar presentation to adult botulism except lack of GI symptoms and longer incubation
• Treatment is combination of surgical management, anti-toxin therapy, and antibiotics

Botulinum Neurotoxins (BoNTs)
• Botox®, Dysport®, Xeomin®
• Due to differences in duration, dose, efficacy, immunogenicity, BoNTs are not interchangeable
• FDA approved indications: Cosmetic use, axillary hyperhidrosis, chronic migraine, neurogenic detrusor overactivity
Clostridium tetani (Tetanus)
• Follows puncture wound contaminated with spores
– Spores germinate in anaerobic conditions
• C. tetani spores are found in soil and animal feces
• Neurotoxin called tetanospasmin that cause sustained contractions of skeletal muscles by inhibition of GABA and glycine at nerve terminals (spastic paralysis)
- Descending pattern of infection
- The A unit cleaves synaptobrevin so vesicle cannot release GABA and glycine so continued release of Ach
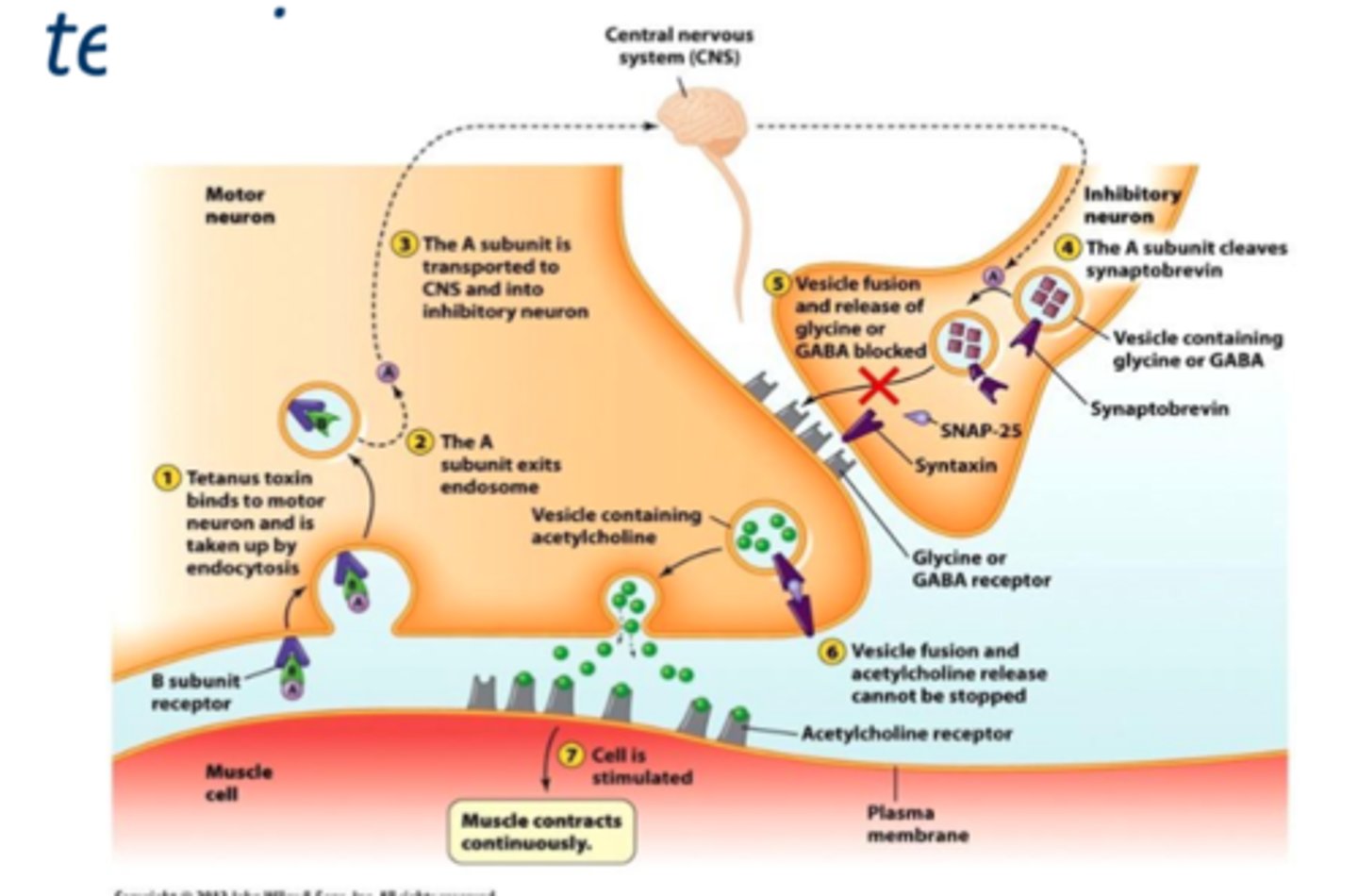
Tetatnus: Vaccine
• DTaP/Tdap
– Reduced diptheria toxoid, tetanus toxoid, and acellular pertussis
– Variable composition and amounts of antigens between products
– DTaP infants and children (< 6 years), Tdap (≥ 11 years)
– DTaP combination vaccinations (IPV, HepB)
– Milk allergy is not a contraindication for use
Tetatunus: More vaccines
• DT
– Vaccination against diphtheria and tetanus
– Up to age 7 years
– Acellular pertussis containing vaccine is contraindicated
– Concentration of diphtheria toxoid is higher in DT vaccine compared to Td
• Td
– Booster vaccination against diphtheria and tetanus
– Age ≥ 7 years
