Chem fundamentals (unit 1)
1.1 The study of chemistry
Matter: physical material; anything with mass that takes up space
Pure substances: matter that has a definite composition, one that does not change, and has distinct properties. They can only be separated by chemical reactions
Elements: pure substances that cannot be decomposed into simpler substances.
Atoms: smallest building block of matter. Each element is composed of 1 type of atom
ex: C
Molecules: 2+ atoms, can be same or different
ex: O2, H2O
Compounds: pure substances composed of 2+ different elements. They can only be separated chemically
Ex: H2O
1.2 Classification of Matter
Property: a characteristic to recognize/distinguish types of matter
Physical properties: can be observed without changing the identity or composition of matter. They are the result of IMFAs between structures (melting point, refractive index, color)
Intensive: properties that are independent of quantity (boiling point, odor)
Extensive: properties that are dependent on quantity (volume, mass)
Chemical properties: observed by destroying substance, they result from chemical reactions
Mixtures: a combo of 2+ pure substances. Each substance maintains its own properties. Mixtures can be separated into its pure substances
Homogeneous: mixtures that are uniform throughout. The components are evenly distributed. They look pure but aren’t since they’re not chemically combined.
Solution: small particles, don’t scatter light
ex: copper sulfate (aq) or brass
Colloid: have large particles, scatter light
ex: milk
Heterogenous: Mixtures that aren’t evenly distributed (granite, wood)
Suspension: you can see layers
1.3 Properties of Matter
Physical change: changes physical appearance, not composition
same substance before and after change
ex: ice→ water (state change)
Chemical change: substance→different substance
Separation of mixtures
Distillation: process depends on the boiling points to form gases
NaCl + H2O: when you boil it, the water evaporates, leaving salt behind
Chromatography: depends on the differing size and polarity of substances to adhere to surfaces of solids to separate mixtures
Separating chlorophyll pigments in leaves
Filtration: mix of solids and liquid that’s poured through filter paper. The liquid passes through, leaving the solid in the filter paper
ex: coffee
2.1: Atomic theory of matter
Dalton’s atomic theory:
Each element is composed of extremely small particles (atoms)
All atoms of a given element are identical to each other
All O2 atoms are the same, all N2 atoms are the same
Atoms of 1 element can’t be changed into atoms of different elements by chemical reactions.
O2 can’t turn into N2
Compounds are formed when atoms of more than 1 element chemically combine.
O + N (elements)→NO (compound)
Law of conservation of mass: matter isn’t created or destroyed, just rearranged
Law of constant composition/definite proportion: given compounds always have same elements in the same proportion. The ratios are fixed
H2O is always 2:1 ratio of H:O
Law of multiple proportions: compounds with different ratios of the same atoms are different
H2O is different from H2O2
2.2 discovery of atomic structure
Democritus: made first atomic model in 400 BC
proposed that all matter is made up of atoms (small, solid, indivisible particles)
Model: ball

Dalton: determined that each element is made up of atoms, created atomic theory
model: ball (same as above image)
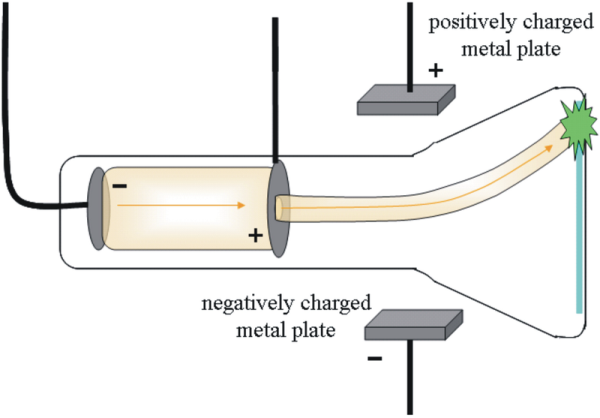
Thompson: through cathode ray tube experiments, determined that there are negatively charged electrons
because electrons contribute a small fractions of atom’s mass, they are small
Cathode ray: A glass tube is pumped with air and when high voltage is applied to the electrodes. radiation occurs between them. The radiation (cathode rays, stream of - charged particles) originate at the anode and travel to the cathode. The rays are the same regardless of the cathode material.
He constructed a cathode ray tube w/ a hole in the anode where the cathode ray can pass. Because the electron is negative, the electric field deflects rays in 1 direction. This allowed him to calculate 1.76 x 10^8 Coulombs/g for the ratio of electron charge: mass
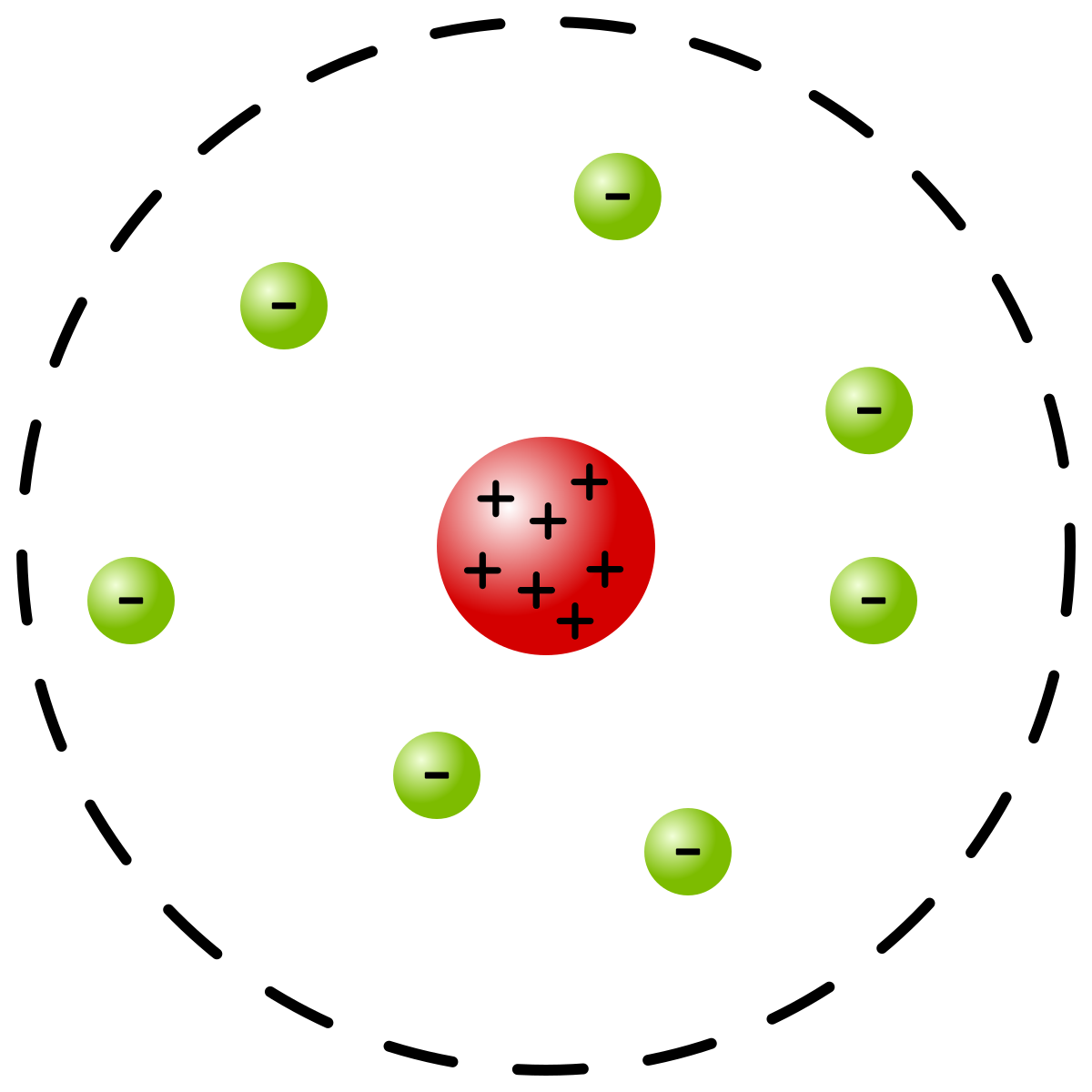
Rutherford: through his gold foil experiment, he discovered protons and the nucleus
Most of the atom’s mass comes from dense + nucleus and most of the volume is empty space (electron cloud)
Bombarded gold foil with alpha particles---majority passed through with no deflection. Very few particles were deflected-----proved that there’s a small but highly charged nucleus
Chadwick: through nuclear bombardment, he found the neutron
Radioactivity: 3 types of radiation (alpha, beta, gamma)
Beta (-1) are high speed electrons bent towards + end
Alpha (+2) bent towards - end
Gamma (0) are high energy waves, no particles or charge bent towards no end, unaffected
2.3: modern view of atomic structure
Nucleus: contains protons and neutrons with an overall + charge.
very small and dense (1x10^-15 m)
Electron cloud: contains negatively charged electrons
almost no mass but most of atom volume (1-5 x10^-10 m)
Angstrom: 1x10^-10m=100pm
Atomic mass unit (amu)=1.66054x10^-24 g
Subatomic particles
Proton: +1, 1.0073amu
Electron: -1, 5.486x10^-4amu
Neutron: 0, 1.0087amu
Isotopic Notation
A: mass number, protons+neutrons
Isotopes: same # protons but different # neutrons, differing mass numbers
Z: atomic number, just protons, used to identify element
q: charge, #protons vs electrons 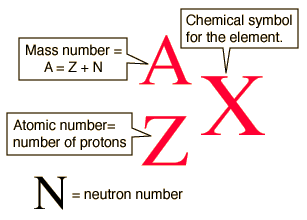
2.4: atomic weights
1amu=1.66054x10^-24 g
1g=6.02x10^23 amu
Atomic mass: the number from the periodic table is dependent on isotopic abundance
Σ (isotope mass)(isotope abundance)
Mass spectrometer:
Get atoms into gas phase and convert them into ions (cations)
When gas phase cations made, they’re accelerated towards negative grid
Only a narrow beam of ions can pass
Beam passes through magnet poles that deflect ions
Ions separated into their masses (isotopes) ---smaller mass goes first
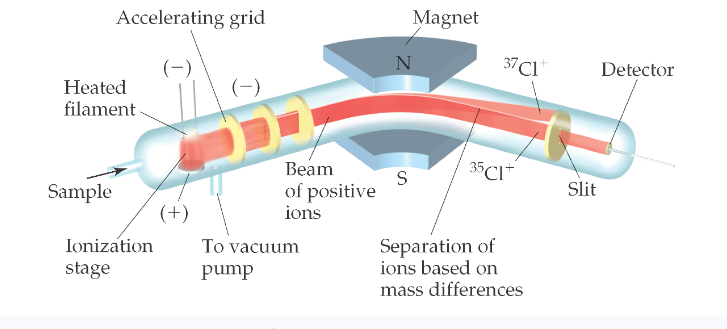
Mass spectroscopy: uses spectrometer to determine the mass of an element/molecule
Provides mass of ions and relative abundance, allows us to calculate atomic mass
converts atoms/molecules into anions to measure mass and abundance in electric field. The beam of ions passes through poles of a magnet which deflects them on a curved path. The extent of deflection depends on the mass. Ions are sorted according to their mass
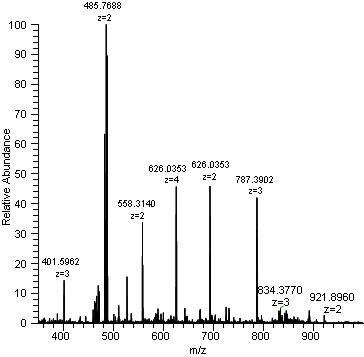
Ex: 9/16 atoms have a mass of 70, 6/16 have a mass of 72, 1/16 have mass of 74
AM=70(9/16)+72(6/16)+74(1/16)=71amu
2.5 Periodic Table
 Periods: the rows
Periods: the rows
Groups: the columns
Group 1: alkali metals
Group 2: alkaline earth metals
Group 6: chalcogens
Group 7: halogens
Group 8: noble gases
2.6 Molecules, molecule compounds
Diatomic: molecules made of 2 atoms naturally (H, O, N, F, Cl, Br, I)
Molecular formula vs empirical formula: molecule is the actual # of atoms in a molecule while empirical is the smallest ratio
Molecular: H2O2 Empirical: HO
Structural formula: drawing that shows how the atoms are joined but doesn’t show geometry
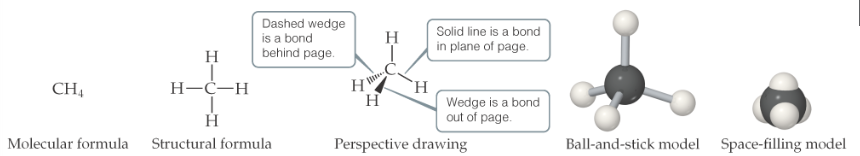
2.7 Ions, ionic compounds
ion: when an atom isn’t neutral, it either lost/gained electrons
cation: positive ion, lost electrons
anion: negative ion, gained electrons
polyatomic ion: atoms joined as one molecule with an overall charge
ionic compound: cations and anions in an alternating crystal lattice. Always in empirical form
formula unit: lowest whole # ratio of cations:anions
3.3 Formula weights
Formula weight: the sum of each atomic weight in a substance
FW of H2O: 2(1.008)+1(16)=18.016 g
% composition: the mass contributed by each element
% comp= #atoms of element (atomic weight) /formula weight x100
Ex: calculate the % composition of C, H, and O for C12H22O11
C: 12(12.01)/342.296 x100=42.10%
H: 22(1.008)/342.296 x100=6.48%
O: 11(16)/342.296 x100=51.42%
Ex: calculate % composition of phosphorus in calcium phosphate Ca3(PO4)2
P: 2(30.97)/310.18 x100=19.96%
3.4: Avogadro’s number
Mole: 6.02x10^23
Calculate the # of H atoms in 0.350 mol of C6H12O6
0.350 mol C6H12O6 | 12 mol H | 6.02x10^23 atoms |
|---|---|---|
1 mol C6H12O6 | 1 mol H |
=2.53x10^24 H atoms
Molar mass: moles of different elements have different masses
1 mole of Cl=35.45 amu but 1 mole of Au is 197amu
Calculate # moles of glucose in 5.380g sample
5.380g C6H12O6 | 1 mole glucose |
|---|---|
180.156 g glucose |
= 0.0298 moles of glucose
Calculate mass of 0.4333 moles of Ca(NO3)2
0.4333 moles Ca(NO3)2 | 164.1 g Ca(NO3)2 |
|---|---|
1 mole Ca(NO3)2 |
=71.06g Ca(NO3)2
5.23 g sample of glucose. Find # molecules
5.23 g glucose | mole glucose | 6.02x10^23 molecules |
|---|---|---|
180.156 g glucose | mole |
=1.75x10^22 molecules glucose
3.5 Empirical formulas
Empirical formula determination--Given percentages of each element
Base calculation on 100.g of compound. It’s easier
Determine # moles of each element for 100.g of compound
Divide each mole value by the smallest mole value to get the ratio
Multiply by the integer to get a whole number formula
Ex: adipic acid has 46.32% C, 43.84% O, 6.85% H
C: 46.32g →4.107 moles C O: 43.84g→2.74 moles O H: 6.85g →6.78 moles H
C: 4.107moles C/2.74 moles=1.50 O:2.74moles/2.74=1.00 H: 6.78moles H/2.74=2.50
Since not whole numbers, multiply everything by 2 →C3H5O2
Ex: Compound is 74% Hg and 26% Cl by mass. Find empirical
74g Hg and 26g Cl
74g Hg(1mole Hg/200.6g Hg)=0.369 moles Hg
26g Cl(1mole Cl/35.45g Cl)=0.733 moles Cl
Hg:0.369moles Hg/0.369 =1 Hg
Cl: 0.733moles Cl/0.369=2 Cl
Empirical: HCl2
Ex: ascorbic acid is 40.92% C, 4.58% H, and 54.50% O. Find empirical formula
40.92g C, 4.58g H, 54.50g O
40.92g C(1mole C/12.01gC)=3.407 moles C
4.58gH(1mole H/1.008g H)=4.54 moles H
54.50g O(1 mole O/16g O)=3.406 moles O
C: 3.407g/3.406=1 C
H: 4.54 moles H/3.406=1.33 H
O: 3.406g O/3.406=1
Empirical: C3H4O3
finding the molecular formula from empirical formulas
whole # multiple=molecular weight/empirical formula weight
find formula mass of empirical formula
Find molar mass of the substance
substance molar mass/empirical molar mass
Multiply the empirical formula by that integer
Ex: empirical formula of adipic acid is C3H5O2 and the molar mass is 146g/mole. What is the molecular formula?
146 g molar mass/73.07 g formula mass =2
2(C3H5O2)= C6H10O4
Ex: C3H4O3 has an experimental mass of 76g.
88.062g C3H4O3 /76g=2
2(C3H4O3)=C6H8O6
Ex: C3H4 has an experimental mass of 121 amu, what’s molecular formula?
121amu/40.062amu=3
3(C3H4)=C9H12
Combustion analysis to find empirical formula
Use mass of CO2 to find the amount of C in organic substance
Use mass of H2O to find amount of H in organic substance
If there’s oxygen in the organic, subtract Cmass and Hmass to get Omass by itself
Once you have masses of each element, proceed like before, get mole substance ratios
Ex: menthol is composed of C, H, and O. A 0.1005g sample combusts, producing 0.2829g CO2 and 0.1159g H2O. What is the empirical formula if the molar mass of the organic is 156g/mole
0.2829g CO2(mole CO2/44.01g CO2)(mole C/mole CO2)(12.01g C/mole C)=0.07715g C
0.1159g H2O(mole H2O/18.016g H2O)(2 moles H/mole H2O)(1.008g H/mole H)=0.01288g H
0.1005-0.0771-0.01288=0.01047g O
C: 0.07715g→0.006429 moles C
H: 0.01288g→0.01288 moles H
O: 0.01047g→0.0006544 moles O
C: 0.006429moles C/0.0006544=10 moles of C
H: 0.01288moles/0.0006544=20 moles of H
O: 0.0006544/0.0006544=1 mole of O
Empirical formula C10 H20 O
Ex:Isopropyl alcohol has C, H, O. The combustion of 0.255g alcohol produces 0.561g CO2 and 0.306g H2O
0.561g CO2(mole CO2/44g CO2)(1 mole C/mole CO2)(/12.01g C/mole C)=0.1531g C
0.306g H2O(mole H2O/18.016g H2O)(2 mole H/mole H2O)(1.008g H/mole H)=0.0342g H
0.255-0.1531-0.0342=0.0677 g O
0.1531g C (mole C/12.01g C)=0.0127moles C
0.0342g H(mole H/1.008g H)=0.0339 moles H
0.0677g O(mole O/16g O)=0.00423 moles O
0.0127moles C/0.00423=3 Carbon
0.0339 moles H/0.00423=8 Hydrogen
Empirical=C3 H8 O
3.6 Quantitative Info from Balanced Equations
Coefficients: the relative # of molecules in a reaction
Ex: 2H2 +O2 →2H2O shows 2 molecules of H2 reacting with 1 molecule of O2 to form 2 molecules H2O
Stoichiometrically equivalent quantities: used to convert between quantities
1.57 mole O2 reactions w H2 to form H2O, how many moles H2 used?
1.57mole O2 x 2mole H2/mole O2 =3.14 mole H2
Determine how many g of H2O produced: C6H12O6 +6O2 →6CO2 +6H2O
1.00g glu x mole glu/180.156g glu x 6 mole H2O/mole glu x 18.016g H2O/mole H2O=0.600g H2O
3.7 Limiting Reactants
Limiting Reactant: the reactant that’s used up first. Once it’s gone, reaction can’t continue
N2 +3H2 →2NH3, 3moles N2, 6 moles H2
3 mole N2 x 2mole NH3/mole N2 =6mol NH3
6mole H2 x2mol NH3/3mole H2=4 mol NH3
LR: H2, RIE: N2, N2 left over: 1 mole
Theoretical and Percent Yields
Theoretical yield: quantity of product calculated to form when all LR is consumed (100% completion, no error)
Experimental yield: amount of product actually obtained (will always be less due to error)
Percent yield: how much you got exp/theo x100%
Percent error: how far you were exp-theo/theo x100%
4.5 Concentrations of Solutions
Concentration: amt of solute dissolved in given quantity of solvent
more solute=more concentrated
Molarity: concentration of moles/L
Calculate M of solution with 23.4g Na2SO4 to make 125ml
23.4g Na2SO4/0.125L =1.32M Na2SO4
What’s M of each ion in 0.025M Ca(NO3)2
0.025mol Ca(NO3)2/L x mole Ca2+/mole Ca(NO3)2=0.025mol Ca2+
0.025mol Ca(NO3)2/L x 2mole NO3-/mole Ca(NO3)2 =0.050 mol NO3-
0.200M HNO3 solution. Calculate moles in 2.0 L
2.0L solution x 0.200mole HNO3/1L =0.40 moles HNO3
Dilution: adding water to make concentration lower
C1V1 = C2V2
1. Want 250 ml of 0.100 M CuSO4 by diluting 1.00 M CuSO4
C1= 1.00 M, V1=? C2=0.100M, V2=0.250L
V1=(0.100M)(0.250L)/1.00M =.0250L or 25 ml of CuSO4 used
2. How many mL of 3.0M H2SO4 for 450mL 0.10M H2SO4
C1=3.00M, V1=? C2=0.10M, V2=0.450L
V1=(0.10M)(0.450L)/3.00M = 0.015 L or 15mL used
4.6 Solution Stoichiometry and Chem Analysis
If we know the solute M, we can used M and V to determine # moles
How many g of Ca(OH)2 to neutralize 25.0mL of 0.100 HNO3 Ca(OH)2 +2HNO3 →Ca(NO3)2 +2H2O
0.025 L x 0.100 mole NO3/L x mole Ca(OH)2/2mole HNO3 x 74.096g/mole Ca(OH)2 =0.092 g Ca(OH)2
Titrations: combining solution with unknown concentration w reagent of known concentration (standard sol)
Equivalence point: where stoich equivalent quantities are brought together
Indicator: dye that changes color as passing equivalence point
1. 45.7mL of 0.5 M H2SO4 req. to neutralize 20.0mL of NaOH sol. What is M of NaOH?
H2SO4 + 2NaOH →2H2O +Na2SO4
45.7mL H2SO4 x L/1000ml x 0.5 mole H2SO4/L =0.229 moles H2SO4
0.229 moles H2SO4 x 2mole NaOH/1mole H2SO4 =0.046 moles NaOH
0.046 moles NaOH/20ml x 1000ml/L =2.285 M NaOH
 Knowt
Knowt
