ap chemistry frq power words
AP Chemistry Unit by Unit Review
Unit 1: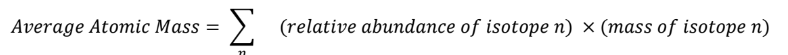
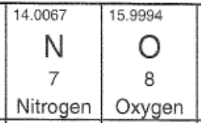
Mass Spectroscopy: measures the abundance of each isotopic mass of an element.
Percent Composition: mass of each element in a compound/total molar mass of substance
Empirical formula steps:
Assume you are given 100g of substance, so change percent to grams
Find moles of each substance
Divide each mole value by the lowest of the mole values (if within 0.1, round)
values found are the subscripts for each element
Molecular formula steps:
Find mass of empirical formula
Divide molar mass by empirical formula mass to find factor
Multiply all subscripts by that value

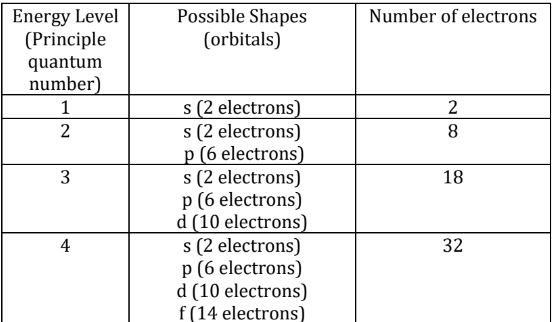
S orbitals: spherically symmetric around the nucleus of the atom, the first orbital
P orbitals: points in a particular direction
D orbitals: a set of five orbitals (with complicated shapes and names) as well as the 3s and 3p orbitals (3px, 3py, 3pz). At the third level there are a total of nine orbitals altogether
F orbitals: seven f orbitals in addition to the 4s, 4p, and 4d orbitals
Coulomb’s Law: the greater the charge of the nucleus, the greater the force is between it and an electron.
Electrons feeling the influence of a more positive charge feel a stronger pull from the nucleus and have lower potential energy.
Aufbau’s Principle: the electron subshells must be filled up in order, “build up”
Hund’s Rule: each subshell must have 1 electron before doubling up
Pauli Exclusion Principle: electrons in the same subshell cannot spin in the same direction and no 2 electrons can have the same 4 quantum numbers
Noble Gas Configuration:

PES Graphs
Binding/ionization energy: the amount of energy needed to remove an electron from an atom
The one with the highest binding energy is the one that is closest to the nucleus due to it having the highest Coulombic attraction.
Periodic Trends
Coulombic Attraction/Electrostatic Interactions: the positive-negative attraction that takes place when you have two charged particles in close proximity
Increases from left to right and as you go up a group
Effective Nuclear Charge/Electron Shielding: the net positive charge experienced by valence electrons
First Ionization Energy: the energy required to remove the outermost (highest energy) electron from the gas of a neutral atom in its ground state
Increases:
Atomic Radius: measure of the size of atoms
Increases:
Electron Affinity: the amount of energy involved when an electron is accepted by a gaseous atom to form a negative ion.
Basically, the neutral atom’s likelihood of gaining an electron
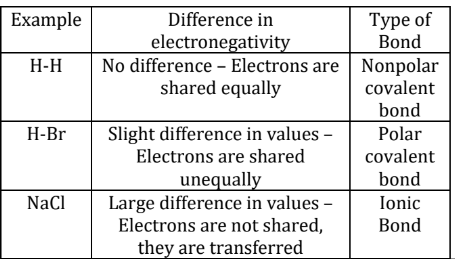
Electronegativity: a measure of the ability of an atom to attract shared electrons
Increases:
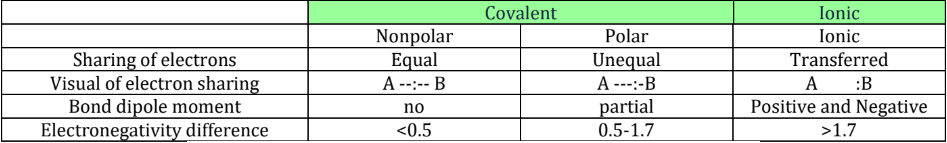
Ionic Bonds: the transfer of electrons from the least electronegative species to the most.
Typically between a metal and a nonmetal
Unit 2:
ION as IC
Occurs between metal and nonmetal when they lose or gain electrons to form ions
Attraction: Coulombic or electrostatic attraction
Ionic bonds are stronger when charges are larger and ions are smaller
Properties
Forms crystals (lattice of positive and negative ions)
High melting/boiling points
Brittle and hard
Conducts electricity when dissolved and molten
Good insulator as a solid
METALLIC
Occurs when multiple metallic cations are attracted to a delocalized sea of valence electrons
IMF is stronger when the cations are smaller and when there are more valence electrons
Properties
Shiny
Malleable and ductile
Conduct heat and electricity
Metal oxides are basic and ionic
Loses electrons to form cations
NONPOLAR AND POLAR COVALENT
Properties
Non-lustrous, various colors
Brittle, hard or soft
Poor conductors
Nonmetal oxides are acidic and covalent
Form anions by gaining electrons
Covalent Bonds:
Bonds between two nonmetals when they share valence electrons
Can be polar or nonpolar, meaning electrons are shared unequally or equally
Can be single, double, or triple bonds (or an average in the case of resonance structures)
Occur at the lowest energy state
Bond Energy: the energy required when breaking a bond, or the energy released when a bond is formed.
Bond Length: larger atomic radii increase the bond length, and longer bond length decreases the bond energy
Increasing the bond order increases the bond energy, as there are more electrons involved and therefore greater coulombic attraction and the bond length has decreased.
Lattice Energy: the change in energy that occurs when gaseous ions are combined to form an ionic solid.
Combining ions will release energy
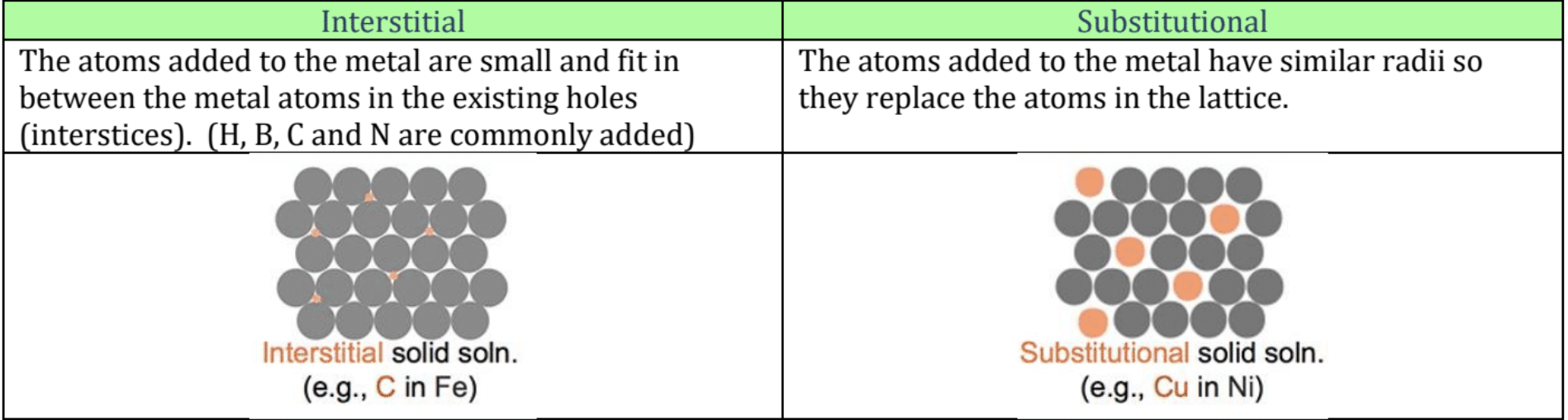
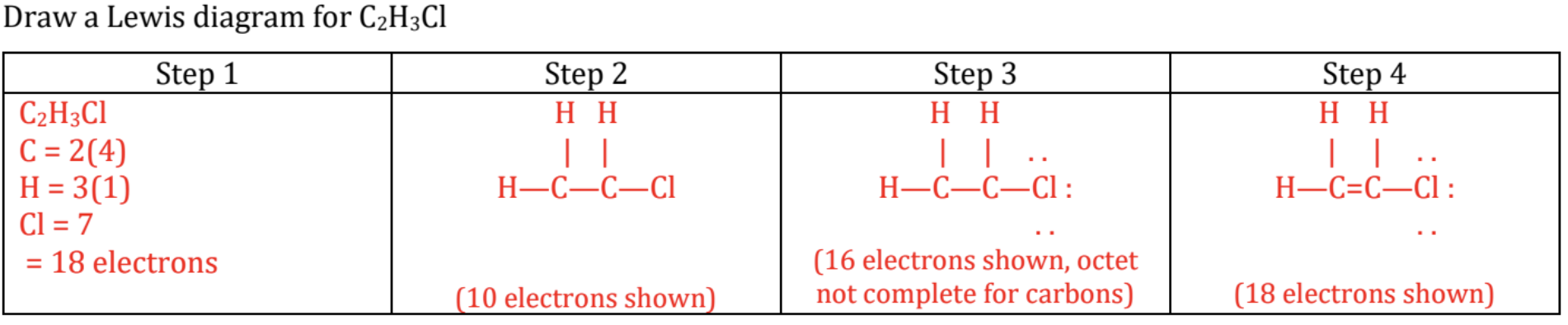
Formal Charge:
How to calculate:
Determine the number of valence electrons of the atom
Subtract the sum of the lone electrons and bonds connected to the atom from the valence electrons
How to check: the sum of the formal charges of each atom should add up to 0 for a molecule, or add up to a charge for a polyatomic ion.
Most valid structure: one that has a formal charge closest to 0 (most preferably “no formal charge”)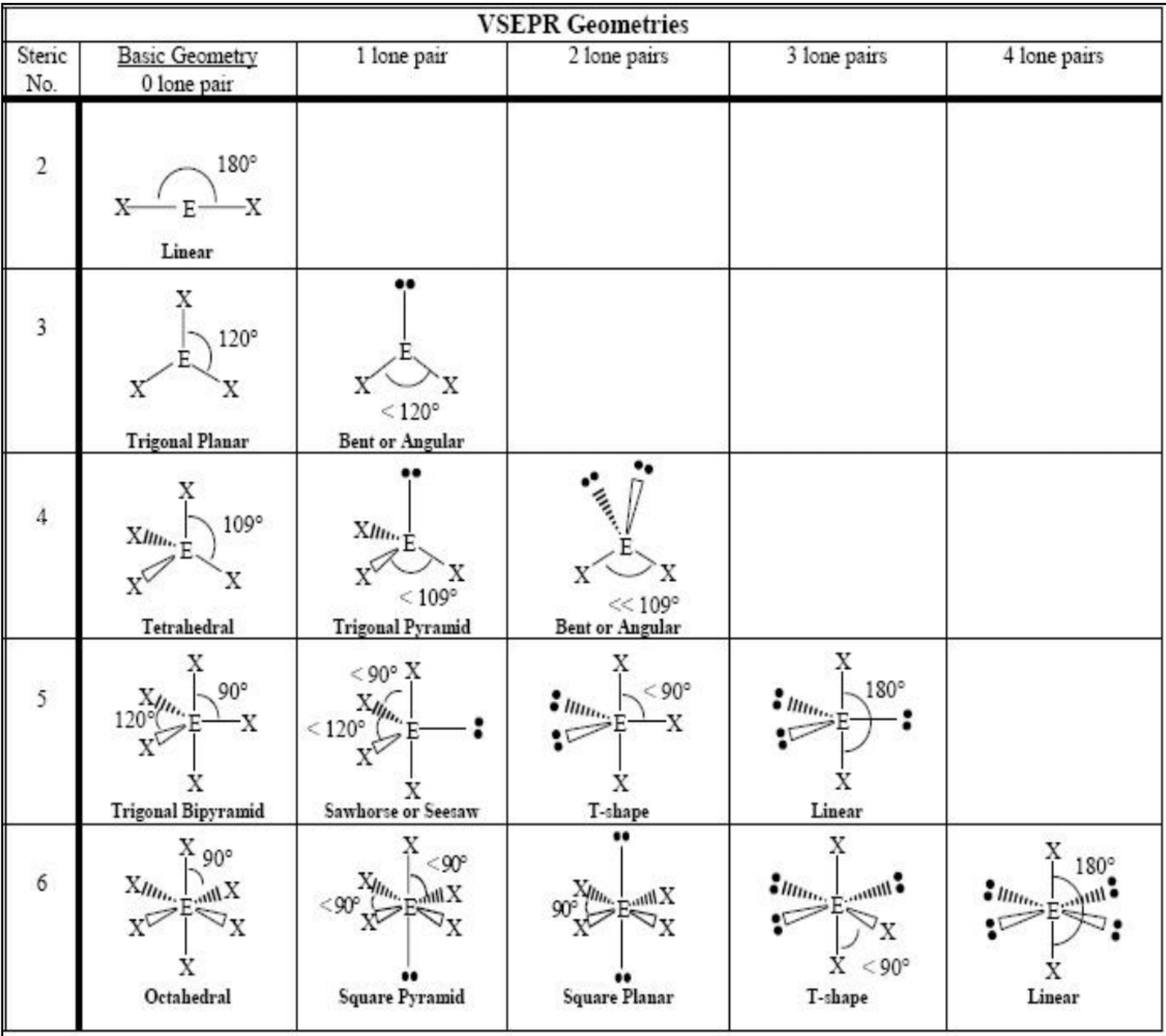
Bond Hybridization:
Linear - 180 degrees - sp
Trigonal planar - 120 degrees - sp^2
Tetrahedral - 109.5 degrees - sp^3
Trigonal bipyramidal - 90 and 120 degrees - sp^3d
Octahedral - 90 degrees - sp^3d^2
Sigma bonds: single bonds
Pi bonds: double and triple bonds, unhybridized p orbitals
Double bonds: 1 sigma bond, 1 pi bond
Triple bonds: 1 sigma bond, 2 pi bonds
Bond Length, Strength, and Energy
As the number of bonds between two atoms increases, the bonds strength and energy increases, but the bond length decreases.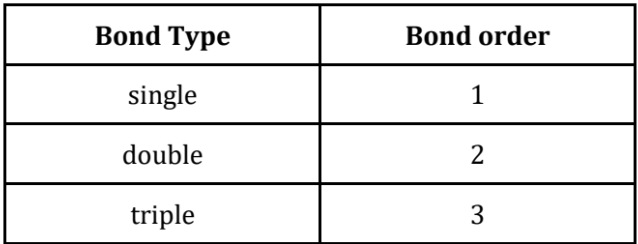
Unit 3:
Polarity:
Covalent bond: electrons are shared between two atoms
However, not all electrons are shared equally
Called a polar covalent bond
Results in a partial electric charge on each atom
Dipoles: a pair of opposite electric charges separated by some distance
Occurs when a polar covalent bond is formed, and the arrow will point towards the atom with the partial positive charge.
Molecular Polarity: depends on the polarity of its bonds and the overall molecular geometry
General rule of thumb: if the central atom has a lone pair and is different from the surrounding atoms, resulting molecule is asymmetric and will be polar
If there are no lone pairs, molecule is symmetric and nonpolar
Intermolecular Forces: what needs to be broken apart in order for covalent substances to change phases
Dipole-Dipole
Occurs between polar molecules: the positive end of one polar molecule is attracted to the negative end of another polar molecule
When molecules have greater polarity, they will have greater dipole-dipole attraction
Molecules with larger dipole moments tend to have higher melting and boiling points
Hydrogen Bonds
Positively charged hydrogen end of a molecule is attracted to the extremely electronegative end of another molecule (ONLY F, O, OR N!!) HYDROGEN BONDING IS FON!!
Much stronger than normal dipole-dipole forces
London Dispersion Forces (LDFs)
Occurs between all molecules
Very weak
Because they depend on random electron movement, molecules with more electrons will have stronger LDFs.
Substances that experience only LDFs will melt and boil at extremely low temps
Tend to be gasses at room temp
STRENGTHS
Strongest: network covalent bonding
Metallic bonding
Hydrogen bonding
Dipole-dipole
Bonding and Phases:
The stronger the IMF, the tighter the atoms are packed together
Solids are highly ordered structures where the atoms are packed tightly together, and they have the strongest IMFs
Gases are spread so far apart and have the weakest IMFs
Ionic substances do not experience IMFs, so instead their phase is determined by the ionic bond holding the substance together in the lattice.
Since they are significantly stronger than IMFs, ionics (typically) = solid @ room temp
Vapor Pressure:
Molecules inside a liquid are in constant motion, so if those molecules hit the surface of the liquid with enough kinetic energy, they can transition from the liquid phase to the gas phase
Known as vaporization
For vaporization to occur, no outside energy is need
Higher the temperature, more likely the molecules break free of IMFs
Solution Separation:
Solutes and Solvents:
*LIKE DISSOLVES LIKE*
Polar solutes will dissolve in polar solvents, and same for nonpolar.
Chromatography: the separation of a mixture by passing it in solution through a medium, in which the components of the solution move at different rates
Paper chromatography:
Rf = (Distance Traveled by Solute)/(Distance traveled by solvent front)
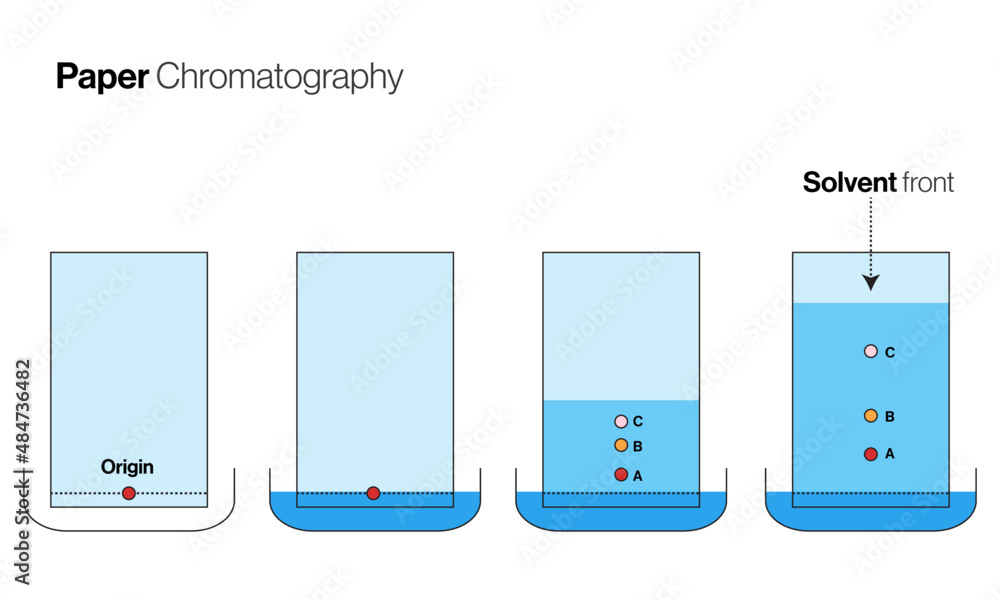
The stronger the attraction between the solute and the solvent front is, the larger the Rf value will be
Column Chromatography:
A column is packed with a stationary substance, and then the analyte (solution to be separated) is injected into the column. Another solution (the eluent) is then injected into the column. As the eleunt makes its way through the column, the analyte will be attracted to it with varying degrees of strength based on their polarity.
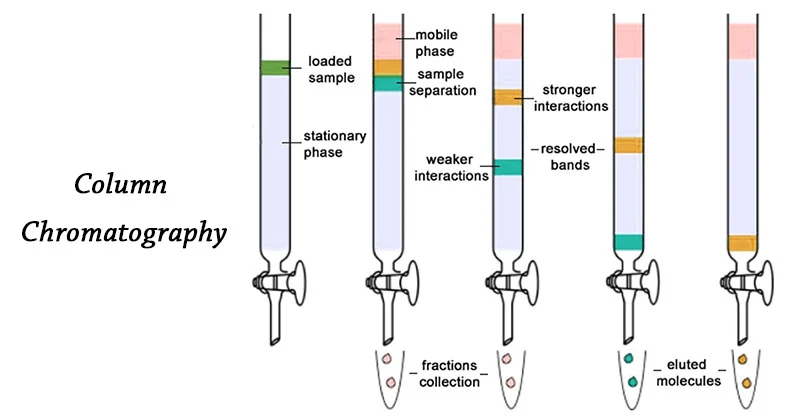
The more attracted the analyte, the faster it will pass through the eluent and exit the column.
Distillation:
Takes advantage of the different boiling points of substances in order to separate them
Kinetic Molecular Theory: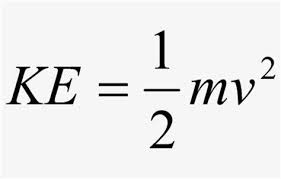
The kinetic energy (KE) of an ideal gas is directly proportional to the temperature:
The higher the temperature, the greater the average kinetic energy
The volume of an ideal gas particle is insignificant compared to the volume in which the gas was contained
There are no forces of attraction between the gas molecules in an ideal gas
Gas molecules are constantly moving and colliding with each other and the walls of their container without losing energy
Maxwell-Boltzmann Diagrams:
Shows the range of velocities for molecules of gas
Takes the average velocity of all the molecules and uses that in the relevant equation to calculate temperature
First type: plots velocity distributions for the molecules of one particular gas at different temperatures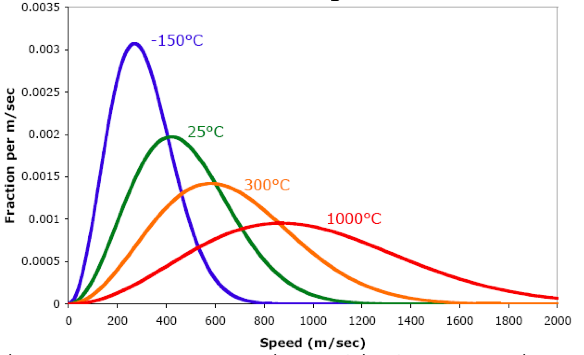
Second type: shows different gases at the same temperature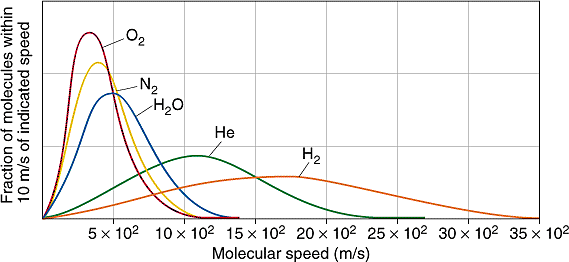
Effusion:
The rate at which particles escape through a microscopic hole in their container, from an area of high pressure to one of lower pressure
Rate of effusion increases with temperature
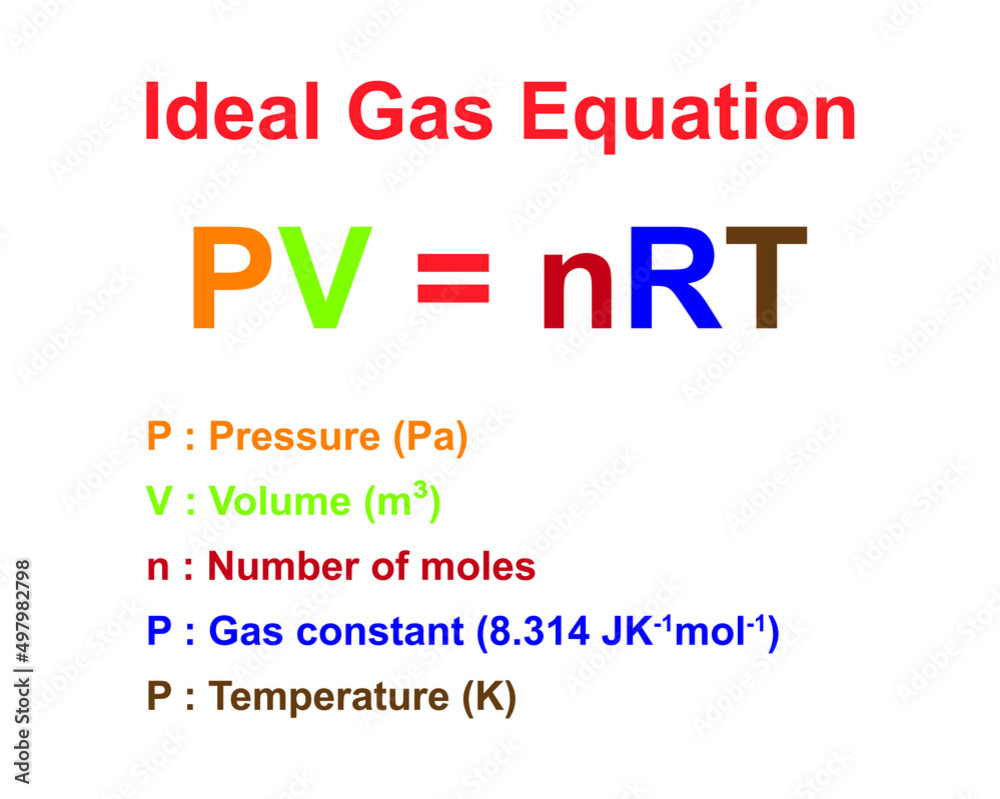
Ideal Gas Equation:
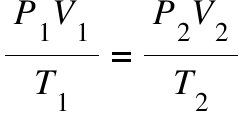
Combined Gas Law:
Dalton’s Law:
Total pressure of a mixture of gasses is the sum of all the partial pressures of the individual gasses 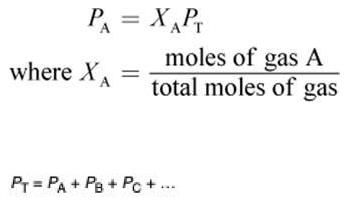
Partial pressure:
Deviations from Ideal Behavior:
When gas molecules are packed too tightly:
The volume of the gas molecules becomes significant
The ideal gas equation doesn’t take into account the volume of the has molecules, but with less free space to move around they become significant
Gas molecules attract each other and stick together
IMFs become significant when packed close together, so some gas molecules will stick together.
Pressure will be smaller than predicted in the ideal gas equation because there are less molecules occupying the space.
Density:
Can be rewritten in many different ways to solve for different things, such as using PV=nRT and replacing V with m/D.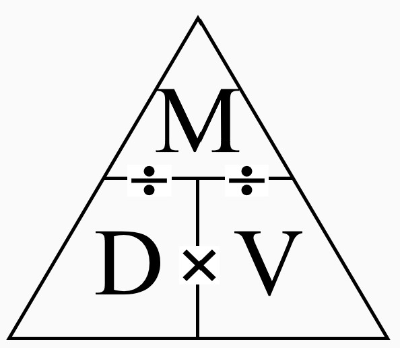
Electromagnetic Spectrum
Energy and Electromagnetic Radiation:
h = Planck’s constant (6.626 x 10^-34 J s)
v = frequency ( in s^-1)
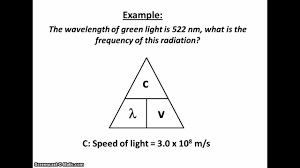
Frequency and Wavelength:
c = speed of light (2.998 x 10^8 m s^-1)
Beer’s Law
Unit 4:
Synthesis Reactions: when elements/compounds are combined to form a single, more complex compound
Decomposition: opposite of synthesis, its when a single compound splits into two or more elements/simple compounds
Acid-Base Reactions: a reaction when an acid (ex. H+) reacts with a base (ex. OH-) to form water and a salt
Oxidation-Reduction (Redox) Reaction: A reaction that results in the change of the oxidation states of some participating species
Cu2+ + 2e- = Cu
Hydrocarbon Combustion: when a covalent substance containing carbon and hydrogen (sometimes oxygen) is ignited, it will react with the O2 in the atmosphere. Products are always CO2 and H2O.
Precipitation: when two (aq) solutions mis, sometimes a new cation/anion pairing can create an insoluble salt.
Can also be written as a net ionic equation, as some “spectator ions” do not play a part in the actual reaction.
SOLUBILITY RULES:
Compounds with an alkali metal cation (Na+, Li+, K+, etc.) or an ammonium cation (NH4+) are always soluble
Compounds with a nitrate (NO3-) anion are always soluble
IONIC SUBSTANCES THAT DISSOLVE IN WATER DO SO BECAUSE THE ATTRACTION OF THE IONS TO THE DIPOLES OF THE WATER MOLECULES EXCEEDS THE ATTRACTION OF THE IONS TO EACH OTHER
Balancing Chemical Equations
*use a trick called backsolving, where you simply take the answers/coefficients they give you, start at a middle number, and start to eliminate from there.
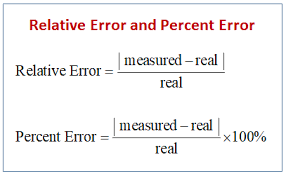
Percent Error
Oxidation States:
Rules:
Any neutral atom not bonded to atoms of any other element has an oxidation state of 0.
Ex: O2 = 0, Cu = 0
Any monoatomic ion has an oxidation state equal to the charge on that ion
Ex: Zn2+ = +2, FeCl3 = iron is +3 and chlorine is -1
In most compounds, oxygen is -2.
Exception: in hydrogen peroxide (H2O2), oxygen = -1
When bonded to a nonmetal, hydrogen = +1
In the absence of oxygen, the most electronegative element in a compound will take on an oxidation state equal to its most common charge
In CF4, fluorine = -1
Combined oxidation states on all elements in a neutral compound must add up to zero. If a polyatomic ion, must add up to the charge
Oxidation-Reduction Reactions
OIL RIG
Acids and Bases
Bronsted and Lowry: defined an acid as a substance capable of donating a proton (H+) and a base as a substance capable of accepting a proton.
Acid: H+
Base: OH-
Unit 5
Rate Law Using Initial Concentrations
Rate Law: the dependence of the initial rate of a reaction on the concentrations of its reactants
Cannot be determined from a balanced chemical equation, must be through experimental data
Found by finding 2 experiments where one of the reactant’s concentration changed, but the rest stayed the same. Look at the initial rate of formation of the product, and see by how much it has increased through division. You get it…
Then, you can plug in one of the equation’s data, as you put the initial rate of formation on top, and then plug in the values for the rate law you just found. DON’T FORGET THE CORRECT UNITS
Rate Law Using Concentration and Time
0th order:
Does not depend on the concentration of reactants at all, so the rate of a zero-order reaction will always be the same at a given temperature
Rate = k
Graph: change in concentration vs time is always a straight line
1st order:
Rate depends on the concentration of a single reactant raised to the first power
Rate = k [A]
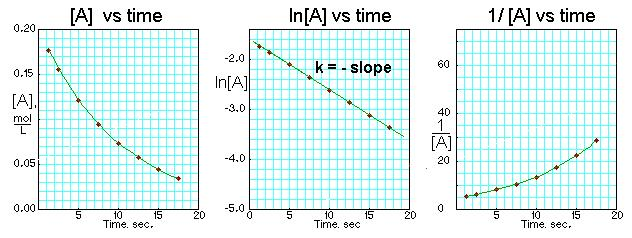
2nd order:
Rate depends on the concentration of a single reactant raised to the second power
Rate = k [A]^2
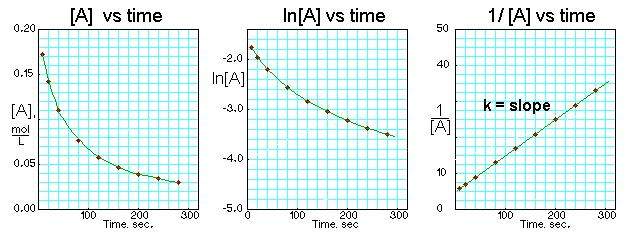
Half-Life
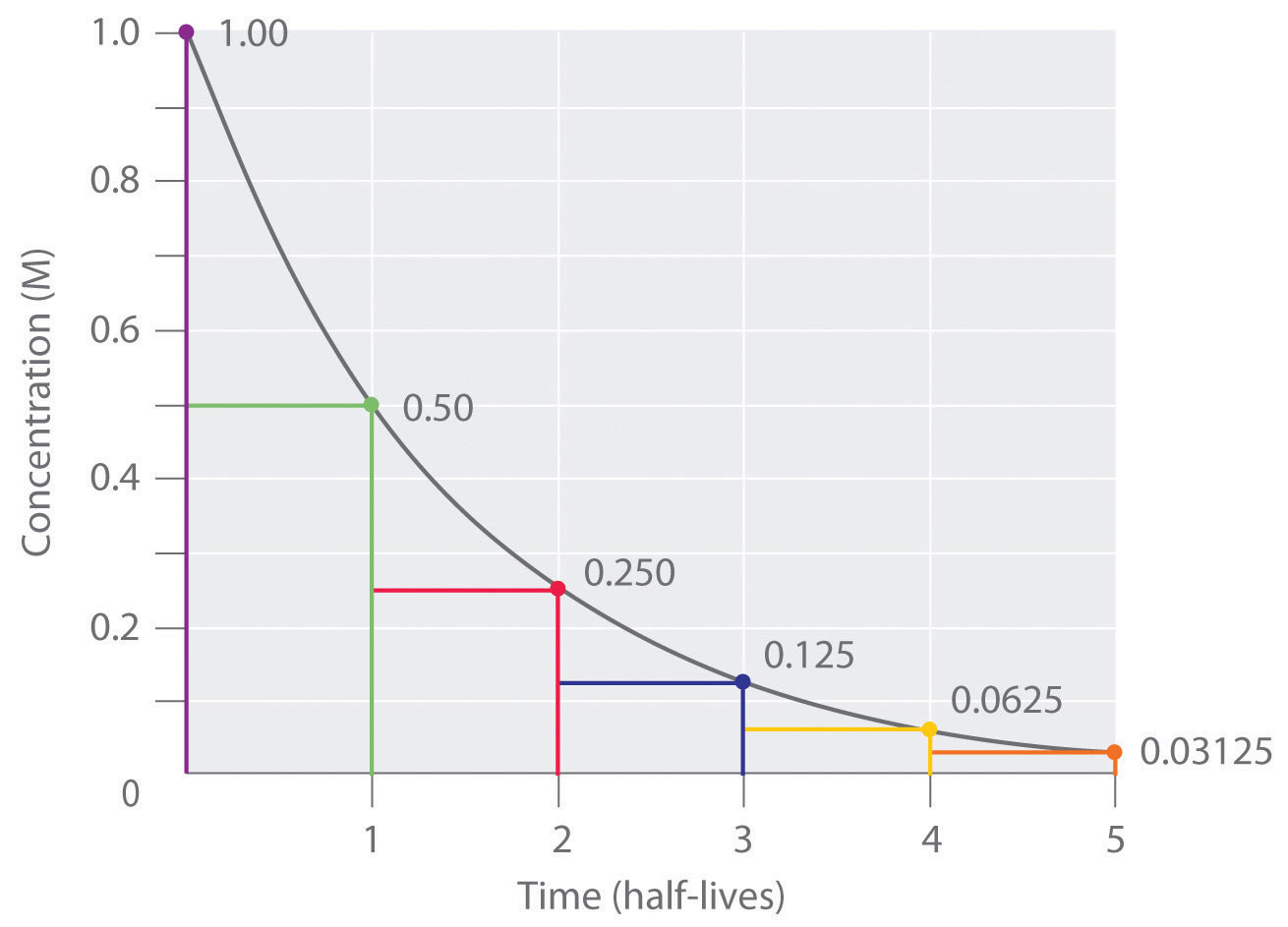
The time it takes for half of the substance to react
For first order reactions, half-life remains constant
Use this equation: half-life = 0.693/k
For zero and second order reactions, half-life is not constant
Cannot use the above equation
Collision Theory
Collision Theory: says that chemical reactions occur because reactants are constantly moving around and colliding with one another
When reactants collide with sufficient energy (activation energy (Ea)), a reaction occurs
Referred to as effective collisions because they lead to a chemical reaction
For gaseous or aqueous reactants, an increased concentration will increase the rate of reaction
Since more molecules are moving around in a given volume, they are more likely to collide
If once of the reactants is a solid, the reaction will proceed faster if the surface area able to react is larger.
Reaction rates increase with increasing temperature because increasing temperature means the molecules are moving faster, when then means that the molecules have greater average kinetic energy.
The higher the temp, the greater the number of reactant molecules colliding with each other with enough energy to cause a reaction
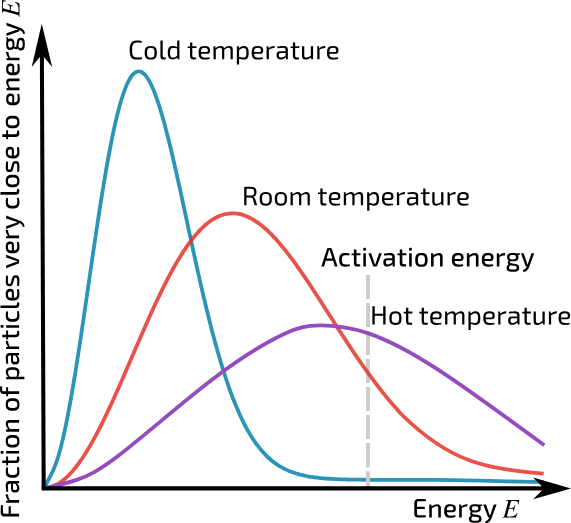
Shown in a Maxwell-Boltzmann:
Reaction only occur of the reactants collide with the correct orientation
Reaction Energy Profile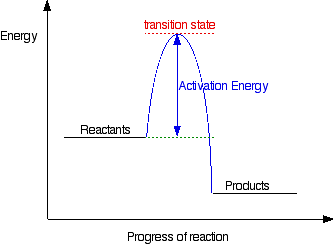
Reaction Mechanisms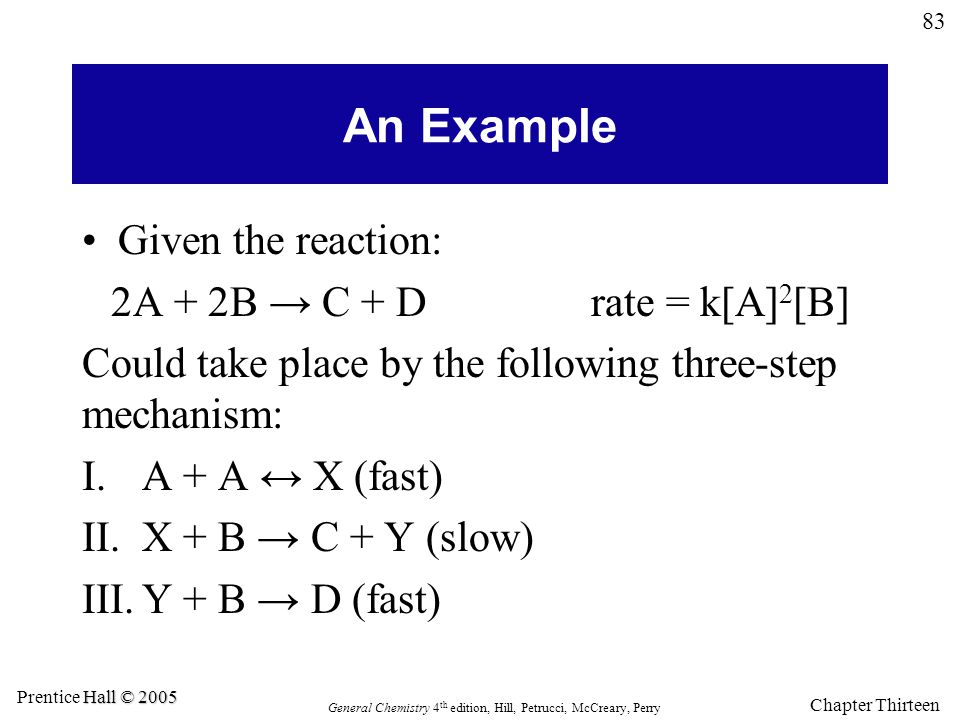
Intermediates: X and Y
Rate-determining step: step 2, as the slow step is always the rate-determining one
We can write the overall rate law using this step, but we have to replace the intermediate with a reactant first. Since we know that A+A=X, we can replace X with 2A. That makes Step II: 2A+B =C +Y
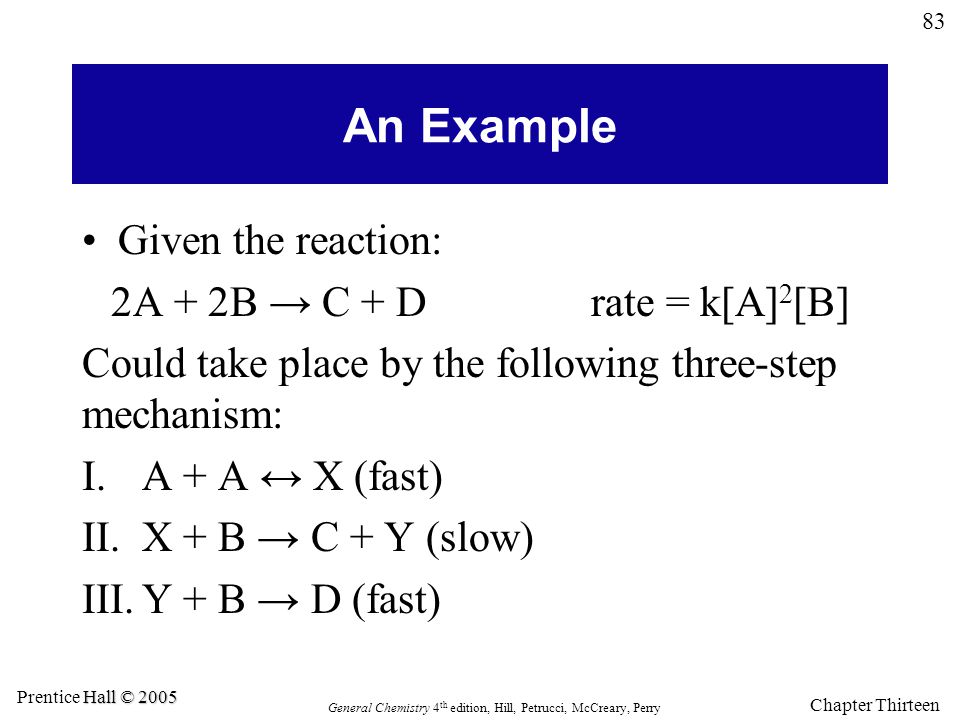
Means the rate law for this reaction is
Important note: you can only use the coefficients to determine the rate law from elementary steps, not from the overall balanced equation
Catalysts
A catalyst increases the rate of a chemical reaction without being consumed in the process, and they do not appear in the overall balanced equation
Catalysts are needed in some cases because otherwise, the reaction would proceed to slowly to be useful at all
Unit 6
Heat and Temperature
Temperature: the average amount of kinetic energy due to molecular motion in a given substance
Heat: energy flow between two substances at different temperatures
Exothermic Process: energy is transferred from the system to the surroundings
If you dissolve a salt in water and the temperature of the water increases, its because the system itself (the dissolving salt) emitted energy. So, the surroundings (the water) absorbed it.
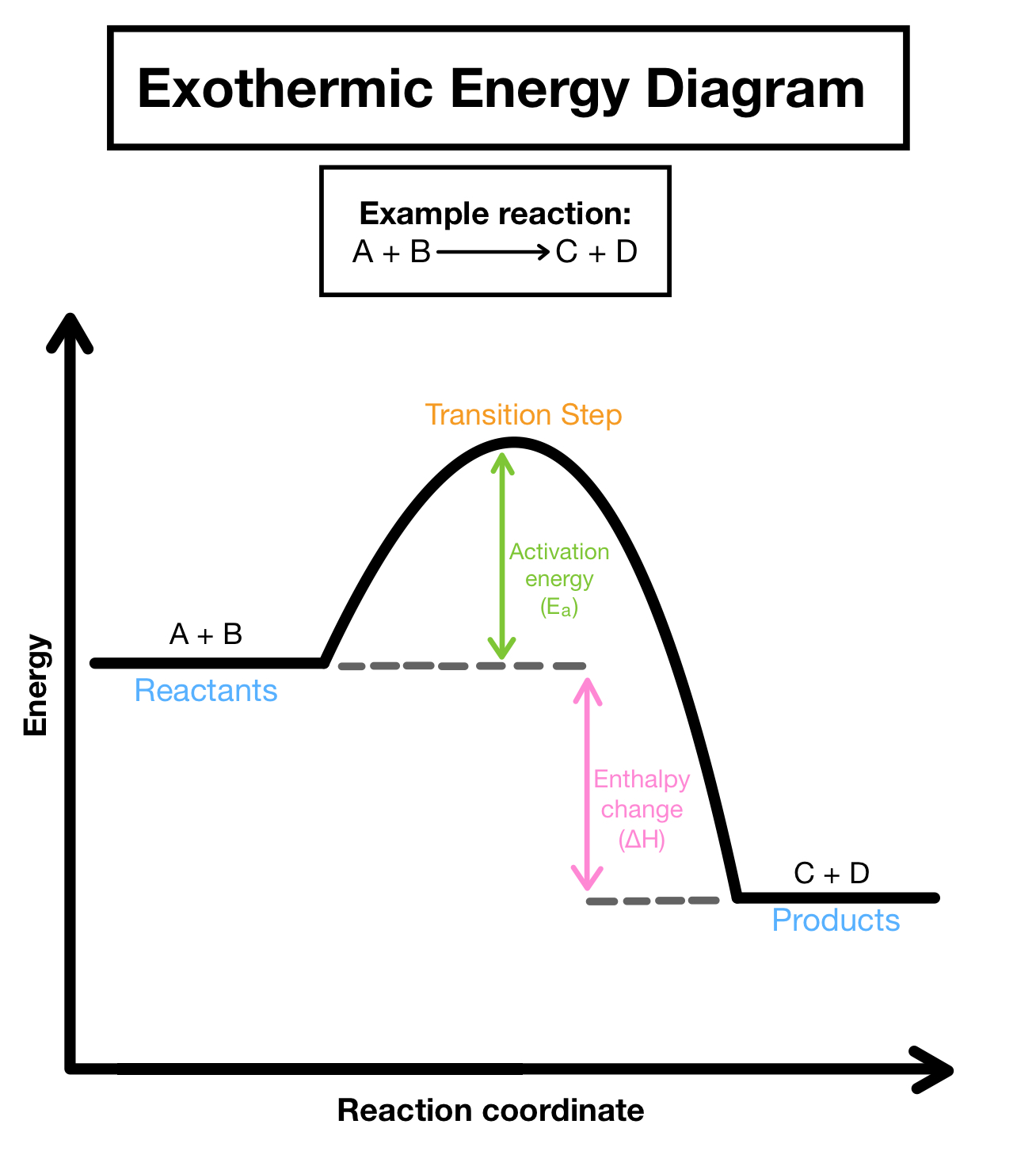
Endothermic Process: energy is transferred from the surroundings to the system
When an ice cube melts in your hand, the energy leaves your hand (the surroundings) and goes into the ice cube (the system)
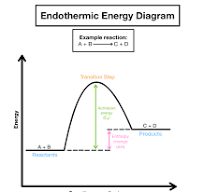
Enthalpy Change, ΔH
When bonds are formed, energy is released.
When bonds are broken, energy is absorbed.
If more energy is released when the bonds in products form than is necessary to break the bonds in reactants, the reaction is exothermic.
Exothermic reactions have a NEGATIVE enthalpy change
If more energy is required to break bonds in the reactants than is released when the bonds form, the overall reaction requires energy from the surroundings and is called endothermic.
Endothermic reactions have a POSITIVE enthalpy change
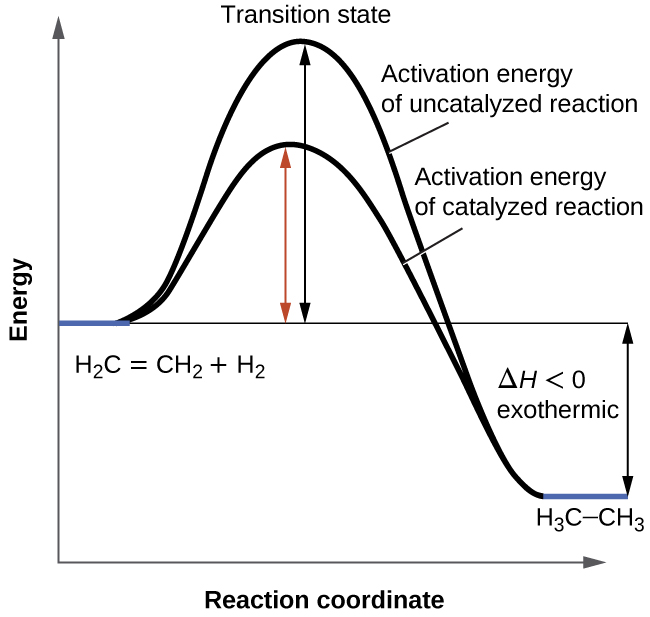
Catalysts and Energy Diagrams
Catalysts speed up the reaction by providing alternate pathways for reactions to proceed that require a lower activation energy or lowering the activation energy of an existing pathway.
Enthalpy of Formation, ΔH°f
Enthalpy of Formation (a.k.a. heat of formation): the change in energy that takes place when one mole of a compound is formed from its component pure elements under standard state conditions
In an enthalpy of formation equation, there is always exactly one mole of product.
This might mean if there is an odd number of a diatomic element in the product, you have to use ½ as a coefficient for that diatomic element. BUT, you cannot use other fractions, and ½ can ONLY be used for DIATOMIC ELEMENTS.
If heat of formation for a compound is negative, energy is released when the compound is formed from pure elements, and the product is more stable than its constituent elements. This means the reaction is exothermic.
If the heat of formation for a compound is positive, that means energy is absorbed when the compound is formed from pure elements, and the product is less stable than its constituents.
ΔH° = ΣΔH°f products - ΣΔH°f reactants
Bond Energy
Basket Ball is My Best Friend
Bonds Broken Minus Bonds Formed
Hess’s Law
Hess’s Law: states that if a reaction can be described as a series of steps, then ∆H for the overall reaction is simply the sum of the ∆H values for all the steps
Enthalpy of Solution
When an ionic substance dissolves in water, a certain amount of heat is emitted or absorbed.
Energy is absorbed because bonds are breaking, but energy is also emitted as dipoles are forming with the water
Step 1: the bonds between the cation and anion must be broken, which always has a positive ΔH
Step 2: the water molecules have to spread out to make room for the ions that just broke apart, and that requires energy to weaken the IMFs between them, so it always has a positive ΔH
Step 3: Free floating ions become attracted to the dipoles of the water molecules, and so energy is being released. Negative ΔH.
The hydration energy is the combined energy values from steps 2 and 3. It is always negative, as the energy change in step 3 is always greater than that in step 2.
If you add all the energy values together, you can determine the enthalpy of solution for that compound.
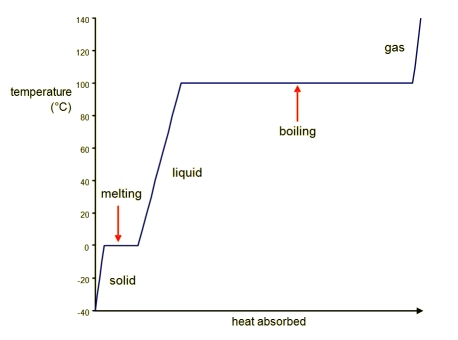
Naming the Phase Changes
Solid to liquid - melting
Liquid to solid - freezing
Liquid to gas - vaporization
Gas to liquid - condensation
Solid to gas - sublimation
Gas to solid - deposition
Enthalpy of Fusion
Enthalpy of fusion: the energy that must be put into a solid to melt it.
This energy is needed to overcome the forces holding the solid together
The heat of fusion, on the other hand, is the heat given off by a substance when it freezes
Enthalpy of Vaporization
Enthalpy of vaporization: the energy that it takes to turn a liquid into a gas.
This energy is needed to overcome the forces holding the liquid together
The heat of vaporization, alternatively, is the heat given off by a substance when it condenses
Calorimetry
Specific heat: the amount of heat required to raise the temperature of one gram of a substance by one degree Celsius (or one Kelvin).
A substance with a large specific heat can absorb a lot of heat without undergoing much temperature change, while a substance with a low specific heat would experience much greater temperature changes
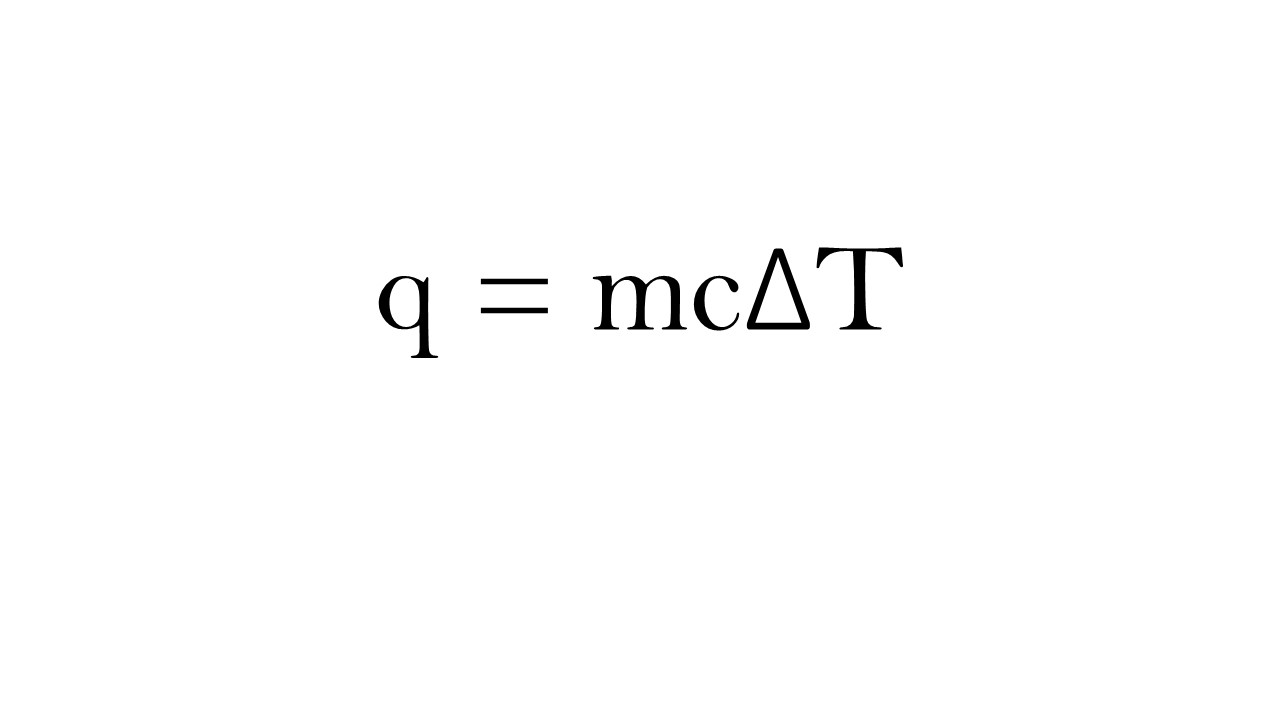
Calorimetry: the measurement of heat changes during chemical reactions
Heating Curves
Shows what happens to the temperature of a substance as heat is added
Unit 7
The Equilibrium Constant, Keq
Most chemical processes are reversible, R to P and back again
Take a look at the Haber Process: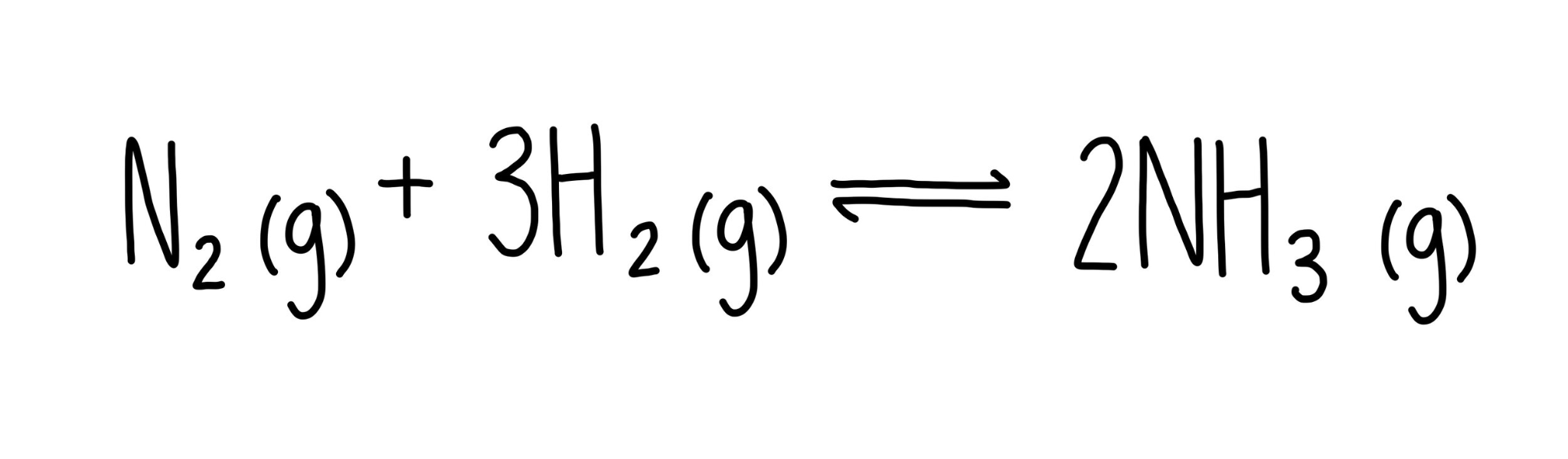
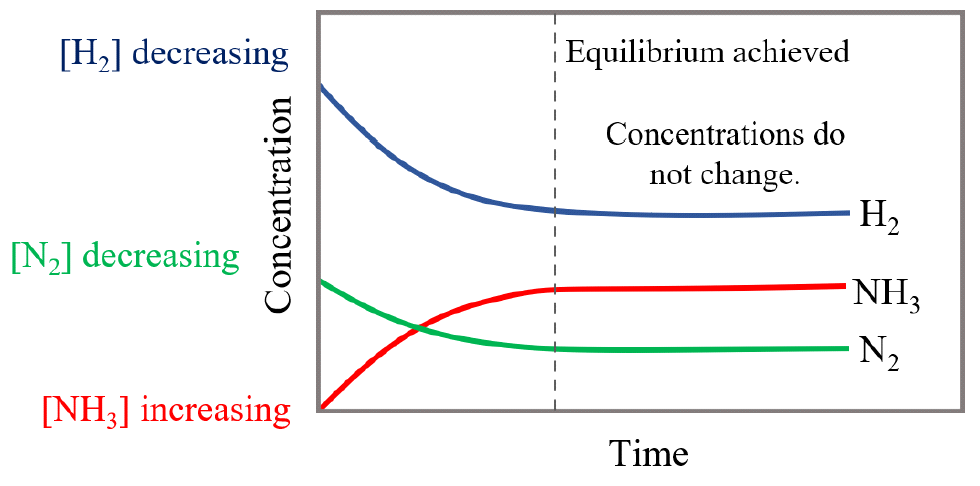
At the equilibrium point, the concentration of all three species stops changing.
Important to mention, because a reaction has met equilibrium does not mean that the reaction is stopped, just that the rates of the forward and reverse reactions are equal
Law of Mass Action: the relationship between the concentration of reactants and products in a reaction at equilibrium is given by the equilibrium expression
The Equilibrium Expression: 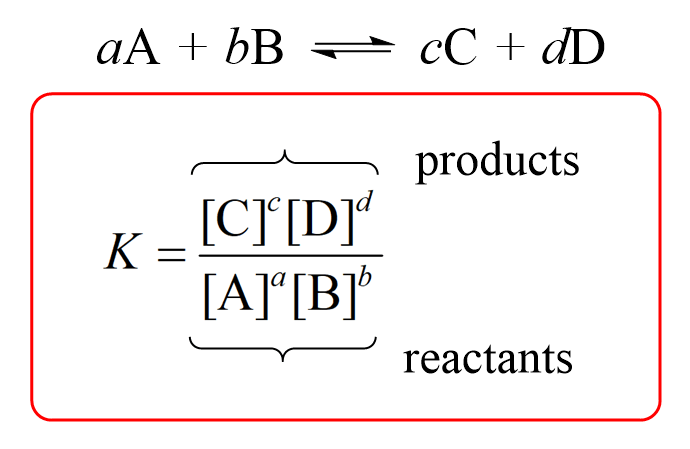
Solids and pure liquids are not included in the equilibrium expression because they cannot change their concentration, only gaseous and aqueous species are included
Equilibrium Constants to be familiar with:
Kc - molar concentration
Kp - partial pressures
Ksp - solubility product (has no denominator because the reactants are solids
Ka - acid dissociation for weak acids
Kb - base dissociation constant for weak bases
Kw - ionization of water (1 x 10^-14)
equilibrium constant: tells you the relative amount of products and reactants at equilibrium
Manipulating Keq
If you flip a reaction, take the reciprocal of the equilibrium constant to get the new one
If you multiply a reaction by a coefficient, you take the equilibrium constant to that power to get the new constant
If you add two reactions together, you multiply the equilibrium constants of those reactions to get the new constant.
Le Chatelier’s Principle
Definition: whenever a stress is placed on a system at equilibrium, the system will shift in response to that stress
Concentration: when the concentration of a reactant or product is increased, the reaction will shift in the direction that allows it to use up the added substance.
Pressure: when external pressure is increased, it will cause a shift towards the side will fewer gas molecules as the partial pressures increase
Temperature: if exothermic, put energy on the products side and vice versa if endothermic. If temp goes up on an exothermic process, the reaction will proceed in a reverse direction to shift away from the added energy
Dilution: if you were to dilute something with extra water, the reaction would shift to the side that has more aqueous species. For evaporation, it would shift towards the side with the less aqueous species
Changes in the Equlibrium Constant
Shifts caused by concentration or pressure change are temporary changes to K, but TEMPERATURE IS THE ONLY THING THAT CAN CHANGE K.
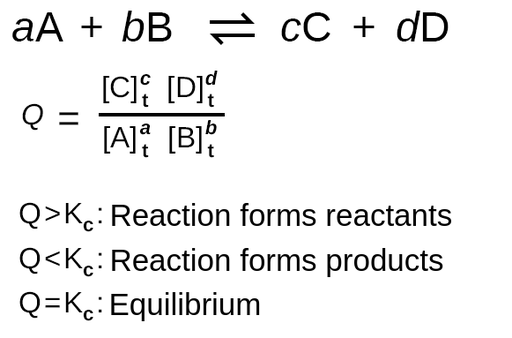
The Reaction Quotient, Q
Essentially a quantitative application of Le Chatelier’s Principle
Solubility
A salt can be considered “soluble” if more than 1 gram of the salt can be dissolved in 100 milliliters of water, but they are usually assumed to dissociate completely in aqueous solution
Solubility Product
Salts that are “slightly soluble” and “insoluble” still dissociate in solution to some extent
The solubility product (Ksp) is a measure of the extent of a salt’s dissociation
The greater the value of the solubility product for a salt, the more soluble the salt.
The Common Ion Effect
Use this equilibrium expression: K = [Ag+] [Cl-] = 1.6 x 10^-10
If we add 0.1 mole of NaCl to the solution, the Cl ions will affect the equilibrium and will disrupt it, even though the Cl- didn’t come from AgCl.
Unit 8
pH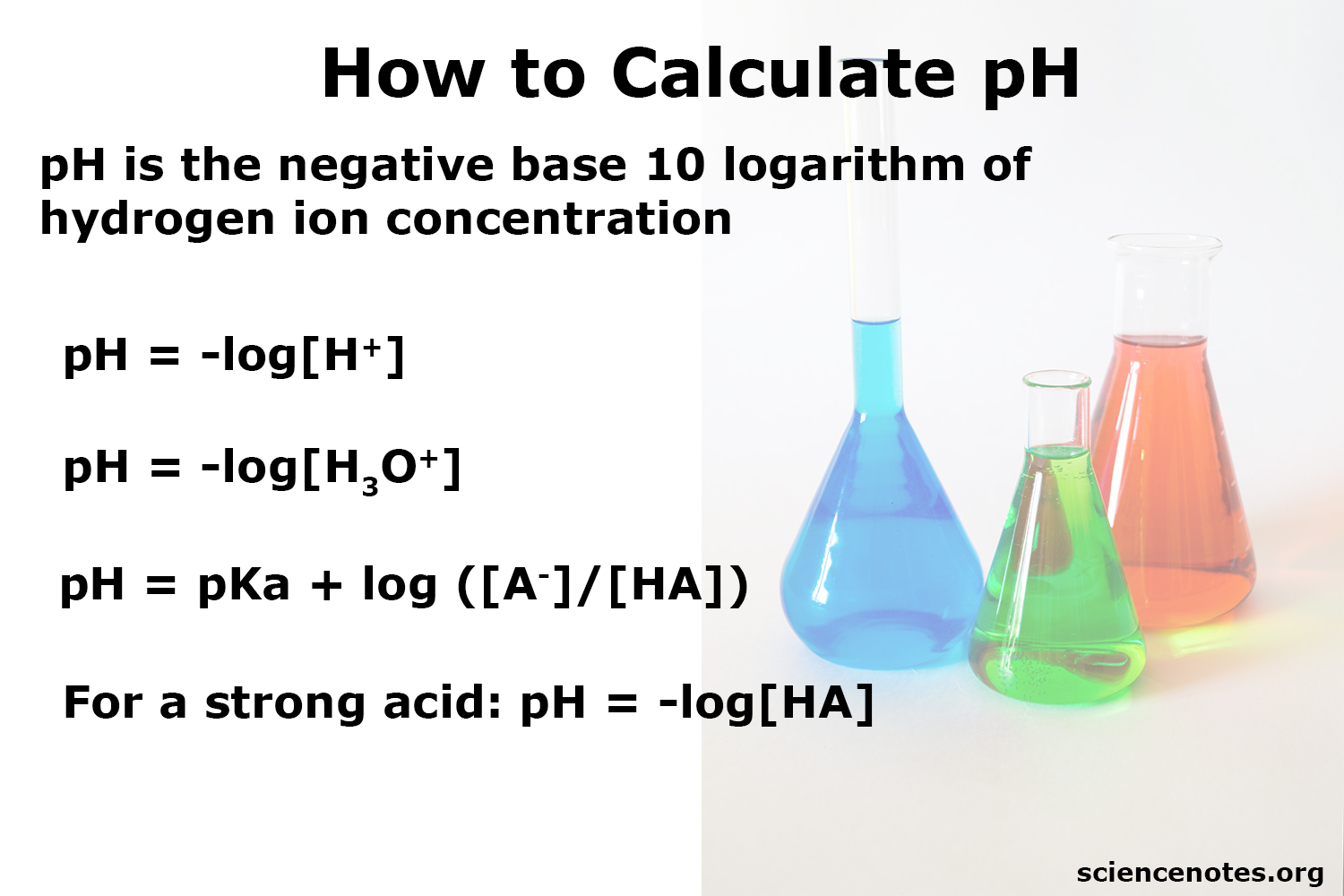
When [H+] = [OH-], the solution is neutral and pH=7
When [H+] is greater than [OH-], the solution is acidic, and the pH is less than 7
When [H+] is less than [OH-], the solution is basic, and the pH is greater than 7
Increasing pH means decreasing H+, as there are fewer H+ ions floating in solution and it is less acidic
Decreasing pH means increasing H+, as there are more H+ ions floating around and the solution is less acidic
ACID STRENGTHS
Strong Acids:
Dissociate completely, so the reaction goes to completion and they never reach equilibrium with their conjugate bases
Important Strong Acids:
HCl
HBr
HI
HNO3
HClO4
H2SO4
There is no tendency for the reverse reaction to occur, which means that the conjugate base of a strong acid must be extremely weak
You can always find the pH of a strong acid directly from its concentration
Weak Acids:
Will not dissociate completely, most acid molecules will remain in solution as undissociated aqueous particles
Need to use an ICE tables to solve for the Ka value of weak acids
For the x that is being subtracted from the initial concentration, we can just disregard it and do x^2/[HA]
x = [H+], so plug that in to the -log equation to find pH
However, if they give the pH and want you to find the Ka value, do not disregard what x is being subtracted from, and instead use the [H+] value you find from the pH and plug it into all of the x spots in the equation
Percent Dissociation
While the strength of an acid is case-specific, it is always true that the easier it is for the H+ ion to break free from the molecule, the stronger the acid will be.
H+ breaks away easier when the element on the other end of the molecule is the most electronegative, as it draws away electrons from the bond with the central atom, which weakens the bond and causes H+ to dissociate more easily.
pH and Solubility
Take manganese hydroxide as an example:
Mg(OH)2 = Mg2+ + 2OH-
Manganese hydroxide would be less soluble in a solution with a high pH than in a solution with a lower pH.
This is because at high pH, there is more OH- in solution, which would shift the equilibrium to the left due to the common ion effect.
Polyprotic Acids
Some acids are able to give up more than one hydrogen ion in solution, but their acid strengths decrease as they give up more and more protons (H+).
Neutralization Reactions
Strong acid + strong base
When these two mix, both substances are dissociated completely and the only important ions in this equation are the hydrogens and hydroxide ions.
Strong acid + weak base
The strong acid will dissociate completely and will donate a proton to the weak base, the product will be the conjugate acid of the weak base
Weak acid + strong base
The strong base will accept protons from the weak acid, and the products are the conjugate base of the weak acid and water.
Weak acid + weak base
This is a simple proton transfer, in which the acid gives protons to the base
Buffers
Buffer: a solution with a very stable pH
You can add acid or base to a buffer solution without greatly affected the pH of the solution, and the pH of the buffer will also remain unchanged if diluted with water or if water is lost through evaporation
Made by mixing a large amount of a weak acid or base into a solution along with its conjugate, in the form of a salt.
****come back to this!
Indicators
Indicators: weak acids which change colors in certain pH ranges due to Le Chatelier’s principle.
Titration
Equivalence point: the point in the titration when exactly enough base has been added to neutralize all the acid that was initially present
Half-equivalence point: the center of the buffer region, where pH = pKa
Unit 9
Entropy: S, measure of randomness or dispersion of the system
The more spread out a system is, the greater the entropy
Since 0 entropy is a solid crystal at 0 K and 0 K has never been reached, entropy will always be positive
Ways entropy increases:
More moles of gas on the products side than the reactants
Goes from solid to liquid or liquid to gas
Goes from one aqueous substance to two aqueous substances
Gibbs free energy: determines whether a process is thermodynamically favored or unfavored
Free energy change:
ΔG° = ΣG°f products - ΣG°f reactants
If negative, thermodynamically favored
If positive, thermodynamically unfavored
If =0, reaction is at equilibrium
ΔG, ΔH, and ΔS
Make sure all are in the same units, one might be in kJ and the other in J!!!
ΔH | ΔS | Temperature | ΔG < 0, thermodynamically favored |
|---|---|---|---|
- | + | all | yes |
+ | - | none | no |
+ | + | High temperature | Only at high temperature |
- | - | Low temperature | Only at low temperature |
Galvanic/Voltaic and Electrolytic Cells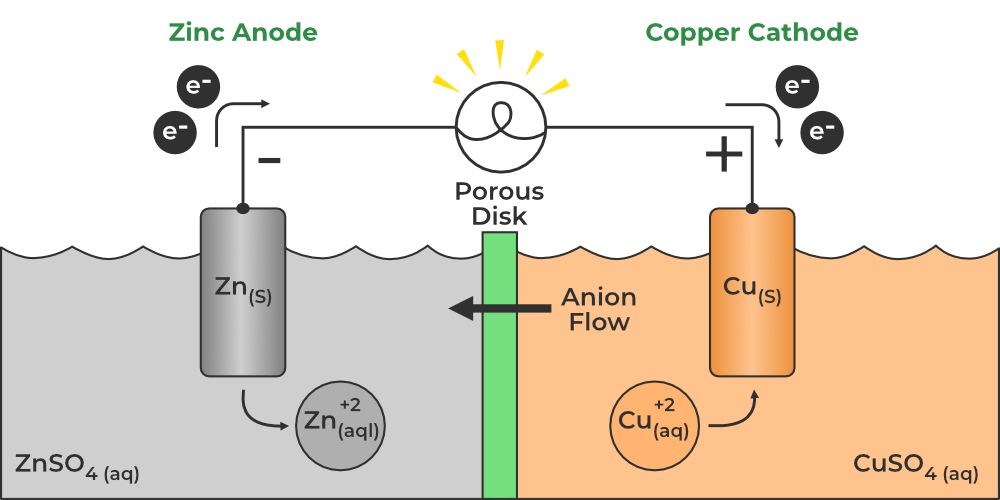
Galvanic/Voltaic:
E°cell > 0
ΔG° < 0
Thermodynamically favorable/spontaneous
K > 1
Has something to measure power
Voltage meter, lightbulb, etc.
Salt bridge: maintains the electrical neutrality in the cell
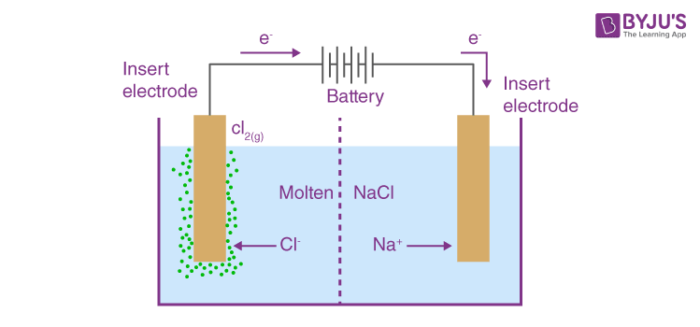
Electrolytic (Electrolysis):
Thermodynamically unfavorable
E°cell < 0
K < 1
Needs a power source to run
Electroplating: electrolytic cells are used for electroplating, have to calculate using the big long conversion factors…
Example of electroplating:
If a current of 2.50 A is run through a solution of iron (III) chloride for 15 minutes, it would cause the following mass of iron to plate out:
15 minutes x x x x x = 0.43 g Fe
Equations:
Q =
Ecell = E°cell - RT/nF lnQ (Nernst Equation)
1 amps = 1 C/1 sec
I = q/t
I = current
E°cell = cathode - anode or reduction potential - oxidation potential
* Red Cat and An Ox
Reduction - cathode
Anode - oxidation
Examples:
10 mol Cu = ?
3 Sn + 2 Au³⁺ → 2 Au + 3 Sn ²⁺
Q =
 Knowt
Knowt
