Lecture 7: Genetics of Antigen Receptors
1/43
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
44 Terms
Basic Requirements of The Immune System
Aim of the immune system is to recognise and remove all the dangers and mount a response to neutralise this all whilst avoiding self-harm through mistargeting/ collateral damage
Innate Immunity
Broad recognition achieved via pattern recognition receptors – recognise PAMPs/MAMPs and DAMPs
They are encoded in the Germline and by a single gene
They are Inherited – evolved to recognise molecular patterns typical of pathogens, microbes’ cells under stress, i.e. danger
Has evolved over the years to recognise these patterns
Adaptive Immunity
BCRs (antibodies) and TCRs recognise specific epitopes of pathogens and bacterium
There are over 20,000 Ab-specific genes, allowing for high specificity.
They are somatically generated via rearrangement of gene segments in developing B and T cells.
These receptor genes are inherited as partially finished segments (gene pools), which are reorganised during development. → somatic generation
This enables a huge diversity of receptors, despite being encoded by relatively few genes.
Features and Implications of Diversity in the Adaptive Immune Receptor Repertoire?
The system generates a highly diverse, random repertoire of receptor specificities.
No prior exposure to pathogens is needed.
Enables broad recognition and makes it hard for pathogens to evade detection.
Risk: autoimmunity – potential development of self-reactive receptors.
Clonal system – each B or T cell expresses only one type of receptor
Clonal Selection
During development, progenitor cells give rise to large numbers of NAÏVE circulating lymphocytes, each with a different specificity
When a lymphocyte recognises an epitope in a dangerous context, this useful clone is expanded to combat the threat.
This also generates MEMORY cells, which will respond faster and better if the same threat is encountered again.
BCRs and TCRs
They are multi-chain receptor complexes with chains that are specialised for recognition and signalling following recognition
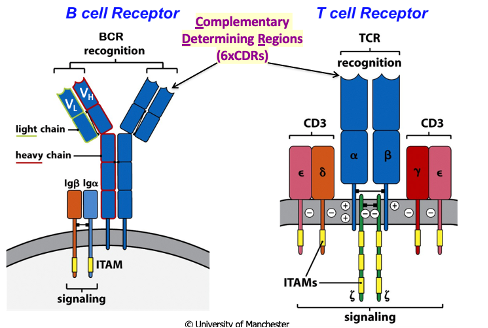
B-Cell Receptor
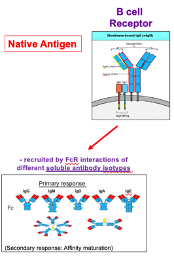
Fab region contains immunoglobulin folds and recognises native, unprocessed antigens.
Upon activation, the receptor becomes a soluble antibody that binds the antigen.
The Fc region mediates effector functions via interactions with Fc receptors (FcRs) on other immune cells.
Different antibody isotypes (e.g., IgG, IgA) engage different effector mechanisms through the Fc region.
T-Cell Receptor
Antigen must be processed, with peptides present in the context of MHC for recognition
Fab region contains immunoglobulin folds
Effector Mechanism: Mediated by cell-to-cell contact or local secretion of cytokines
These receptors mediate their effects locally → don’t need to make secreted forms of the receptor
Structure of Typical Antibody (IgG)
Composed of 2 identical heavy chains and 2 identical light chains.
Light chains can be of two types: kappa or lambda, both functionally similar.
N-terminal ends of both chains have variable regions (at first immunoglobulin domains), followed by constant regions.
In heavy chains, the constant regions differ by isotype (e.g. IgG1).
The antibody forms a Y-shaped structure with Fab regions (antigen binding) and an Fc region (effector functions).
How does the antibody variable region contribute to antigen binding?
Kabat and Wu sequenced many antibody variable regions and analysed amino acid variability.
They identified 3 hypervariable regions (CDRs) responsible for antigen binding.
These regions are located in loops of the variable domain, allowing high variability without disrupting structure.
Framework regions (shown in yellow) are less variable to maintain the structural immunoglobulin fold.
The light chain immunoglobulin fold and heavy chain combine to form a beta-barrel, positioning the hypervariable CDRs to create the antigen-binding site.
Formation of B-Cell Receptor
occurs via lineage-specific, developmentally regulated somatic recombination of gene segments.
Only B cells rearrange immunoglobulin genes to form BCRs;
TCR genes rearrange only in T cells.These gene rearrangements occur at defined developmental checkpoints during lymphocyte development and maturation.
Structure of a Typical Gene
It is inherited as introns and exons in genomic DNA.
It contains a promoter, followed by sequences transcribed until a stop signal, after which a poly-A tail is added.
Splicing machinery removes introns, forming mature mRNA.
The ribosome reads the single open reading frame in the mRNA to synthesise a protein.
Structure of Kappa Light Chain
The constant region is encoded by the C-exon.
The variable region is inherited as separate V and J gene segments (not full exons → incorrect ends).
In genomic DNA, V, J, and C regions are separate.
During B-cell development, as a cell commits to the B-cell lineage, transcription machinery is activated, and epigenetic changes loosen the chromatin around the light chain locus.
Somatic recombination joins a specific V segment (e.g., V36) to a J segment (e.g., J3) to form a functional V exon.
occurs at the level of the genomic DNA
The primary transcript still includes the remaining J segments in an intron, which are removed during mRNA splicing.
The resulting mature spliced mRNA is translated by ribosomes into a kappa light chain protein.
Gene Rearrangement
Process where different B-cells choose different V and J segments through gene re-arrangement
It results in clonal B-cells with light chains that have different antigen-binding properties
Generation of Complementary Determining Region (CDR)
CDR1 and CDR2 are both encoded within the V (variable) kappa gene segment, and their variability depends on which V segment is selected for recombination.
CDR3 spans the V-J junction:
The beginning of CDR3 comes from the V segment,
The end from the J segment,
The join introduces additional variability.
This makes CDR3 the most variable of the CDRs.
Arrangement of Gene Segements at Human Immunoglobulin Gene Loci
Light chains locus located on different chromosomes sit next to each other→ (κ and λ) involve rearrangement of V → J segments on separate chromosomes.
E.g., Vλ2 joins Jλ2, eliminating any DNA in between, forming the V exon and the C-terminus
Heavy chains (IgH) contain an additional D (Diversity) segment → require two rearrangements:
D → J, then V → DJ.
The final rearranged V exon is VDJ.
CDR1 & CDR2 are encoded within the V segment.
CDR3 is formed from the V, D, and J segments, with both joins contributing to its extreme variability.
Combinatorial Diversity in Immunoglobulin Gene Rearrangement
It arises from the random recombination of V, D, and J segments:
Heavy chains (H): 40V × 23D × 6J = ~5520 combinations.
Light chains (κ + λ): 70V × 5J = ~350 combinations.
Heavy + Light chain combinations:
H + L: 5520 × 350 = ~1.9 million combinations.
Joining imprecision: During recombination, variability is introduced due to imprecision at the V-D, D-J, and V-J joins, increasing (junctional) diversity.
How do recombination signal sequences (RSS) and the 12-23 rule guide the V-D-J joining process?
Each gene segment has a recombination signal sequence (RSS):
Heptamer, spacer (12 or 23 base pairs), and nonamer.
The 12-23 rule ensures proper joining V-D-J joining order:
A segment with a 23-base pair spacer will join with a segment that has a 12-base pair spacer.
Enzymes in the recombination machinery recognise these sequences and pull the ends of segments together, resulting in a cleavage near the heptamer, close to the V and J segment
The intervening DNA sequence is removed bringing the segments together to form the V-D-J exon.
Molecular Mechanics of V(D)J Recombination
V and J segments each have a recombination signal sequence (RSS) with 12 and 23 base pair spacers.
As B-cells mature, they activate RAG1 and RAG2 genes, which, along with other proteins, recognise these sequences and pull the segments together.
This causes a cleavage near the heptamer, forming hairpin loops at the coding ends.
The coding ends are recognised as damaged DNA by DNA repair machinery, which nicks the ends to allow the V and J segments to join.
The nicking process and subsequent end-resolution introduce some imprecision at the joining site.
The enzyme TdT (a lymphoid-specific enzyme) adds random nucleotides to the junction, increasing diversity.
Generation of Junctional Diversity Through Imprecise Joining of Gene Segments
RAG enzymes bind to D and J segments, pulling them together and cleaving the RSS heptamer, forming coding ends.
Coding ends are nicked and unfolded.
Palindromic P nucleotides are added as the DNA is unwound and is palindromic.
TdT adds N-nucleotides (random nucleotides) to the junction.
The strands pair and align until a good match is achieved.
Exonucleases trim and fill in the ends to form the coding joins.
P-nucleotides are present at the ends, contributing variability to CDR3.
Imprecision in the process, like random codon insertions, can lead to frameshifts or non-productive joins (e.g., stop codons, out-of-frame joins).
Combinatorial Diveristy
Source of diversity in the primary antibody repertoire
Multiple gene segments
any H with any L
Junctional Diveristy
Source of diversity in the primary antibody repertoire
P-nucleotide addition
N-nucleotide addition
exonuclease activity – removal of things out
most of this diversity is localised at the CDR3 joins
T-Cell Development
Cells develop in the bone marrow with the progenitors moving to the thymus to make alpha beta and gamma delta t-cells
alpha/beta (ab) T-cells:
most abundant type of T-cell (~95%)
gamma/delta (g/d) T-cells:
play roles in immune responses at epithelial surfaces. Innate?
How are the TCR alpha and beta chains encoded in the germline, and how do they compare to antibody chains?
TCR gene loci consist of multiple gene segments.
The TCR alpha chain is similar to the immunoglobulin light chain:
Formed from V and J segments that together make the V-exon.
The TCR beta chain is similar to the immunoglobulin heavy chain:
Includes D, J, and V segments.
Rearrangement occurs in two steps: D + J, then V → DJ to form the functional beta chain
TCR Gene Segment Recombination
TCR gene segments are flanked by Recombination Signal Sequences (RSSs).
These RSSs are recognised by the same lymphoid-specific RAG1 and RAG2 proteins used in B cells.
T and B cells share a similar gene organisation and recombination mechanism.
Epigenetic changes loosen chromatin around the TCR α and β loci to allow recombination machinery access.
B cell receptor genes recombine in B cells during development in the bone marrow.
T cell receptor genes recombine in T cells during development in the thymus.
Comparison of Diversity Sources in Immunoglobulins vs. αβ TCR
TCRs exhibit greater junctional diversity than immunoglobulins.
Both immunoglobulins and TCRs incorporate P-nucleotides at coding joins (due to hairpin resolution).
TdT (Terminal deoxynucleotidyl Transferase) adds N-nucleotides:
Terminal deoxynucleotidyl Transferase (TdT)
It is active during heavy chain rearrangement, contributing to greater variability in heavy chain joins
Its activity decreases during light chain rearrangement, leading to reduced variability in light chain joins
Turned on during both α and β chain rearrangements in T cells → adds N-nucleotide diversity in both chains.
Difference in Reading Frame flexibility in D segments between TCR β chains and immunoglobulin heavy chains
TCR β chains: D segments can be read in 3 frames, and with 2 recombination joins (V-D & D-J), there's more flexibility to stay in-frame → higher chance of producing a functional receptor.
Ig heavy chains: Also have 3 potential reading frames, but out-of-frame joins often lead to stop codons, resulting in non-productive rearrangements.
Overall: TCR β chains allow for more diversity without loss of function.
Sources and Distributions of Diversity between Immunoglobulins and T Cell Receptors
Both use somatic recombination of gene segments → similar level of combinatorial diversity.
TCRs have greater junctional diversity due to:
D segments read in all 3 reading frames.
TdT activity at all junctions.
More J segments in TCRs.
TCR diversity is concentrated in CDR3, which has the main contact with the peptide presented by MHC.
Greater CDR3 diversity in TCRs → directly interacts with the antigenic peptide in the MHC groove.
Clonality in B-Cells
Each B cell inherits:
2 heavy chain alleles
2 light chain kappa alleles
2 light chain lambda alleles
Clonality requires expression of only one heavy and one light chain allele from the parents.
This is enforced by checkpoints through:
Allelic exclusion (prevents both alleles of a gene from being expressed)
Isotypic exclusion (only one type of light chain—kappa or lambda—is used)
Mature Activated B-Cell
Only 1 productively rearranged heavy chain allele is active
Only 1 productively rearranged light chain allele is active
All other alleles are excluded from expression
Gene Rearrangements in B Cell Development
Stepwise, regulated process:
Heavy chain rearrangement starts first:
• D joins to J segment
• Then V joins to DJ to form the V exonIf a productive heavy chain is formed (passes the checkpoint):
• Rearrangement of the other heavy chain allele is stopped
• Light chain allele rearrangement beginsIf the first heavy chain rearrangement is non-productive:
• Rearrangement occurs on the second chromosome
• If still non-productive, the cell is eliminatedLight chain rearrangement:
• V joins to J
• If unsuccessful on the first allele, attempts rearrangement on the second allele
• Must pair with heavy chain to produce a functional antibody on the surface
Heavy Chain Rearrangement in B-Cell Development
D to J, then V-D to J; first checkpoint occurs
If a productive heavy chain is formed, it combines with a surrogate light chain and reaches the surface
Crosslinking with the surrogate light chain signals the cell to produce more heavy chains
RAG genes are active during this phase of recombination
RAG Gene Activity During B-Cell Development
RAG genes are active during the recombination steps
RAG genes temporarily turn off during the proliferation phase
This results in the production of multiple B cells with the same heavy chain but potentially different light chains
Once proliferation finishes, heavy chain genes become inaccessible
Light Chain Rearrangement in B Cell Development
After proliferation, light chain genes become accessible
RAG genes are reactivated and V to J rearrangement occurs
If this doesn't work, rearrangement proceeds on the lambda chain
A functional light chain that can combine with the heavy chain and reaches the surfaces, signals to turn the RAG genes off
TCR Alpha and Beta Chain Rearrangement
The beta chain of the TCR binds with a surrogate alpha chain
TdT is active throughout the rearrangement of the alpha and beta chains
The rearrangement of the alpha chain contributes to the diversity in CBR3
Fate of BCRs Following B-Cell Activation
BCRs are made soluble and become an antibody → recruit effector function by binding with the antigen through the Fc receptor
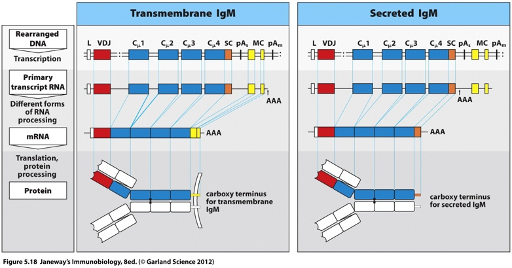
Membrane Bound BCRs
The C-terminal region of the membrane-bound BCR contains cytoplasmic and transmembrane domains, which are encoded by the ‘yellow’ exons.
A splice site between the ‘blue’ exons (coding for the antibody region) and the ‘yellow’ exons allows for alternative splicing to generate the membrane-bound form of the BCR.
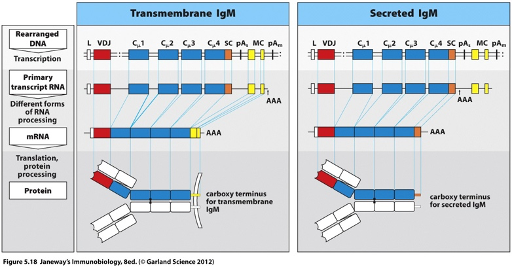
Secreted BCR
Generated by alternative RNA processing.
The C-terminus o is encoded by the blue exon.
Upon B cell activation, an alternative polyadenylation (poly A) site is used, which determines where the polyadenylation signal is added.
The yellow exons (which encode the transmembrane domain) are no longer spliced in.
Instead, the transcript runs through the blue exons to the next stop codon, giving rise to a splice site for the secreted form.
Role of C-Region Exons in Class Switching
Class switching is organised within the heavy chain gene locus.
C-region exons for different isotypes (IgM, IgD, IgG, IgE, IgA) are located 3’ of the JH gene segments.
These C exons are lined up along the genome, allowing a B cell to take its recombined V segment and join it to different C-region exons.
This enables a single B cell clone to switch the antibody isotype it produces, while retaining antigen specificity.
Although each B cell has the potential to express all isotypes, it only expresses one at a time (except for IgM and IgD, which can be co-expressed before class switching).
Co-Expression of IgM and IgD
Occurs early on B-cell development → regulated by alternative RNA processing
The only time that two isotypes are expressed at the same time
If it stops at the polyadenylation site = IgM.
If it uses the other 2nd polyadenylation site=IgD
Mechanism Behind Isotype/ Class Switching in B-Cells
Isotype switching occurs via recombination between switch (S) regions upstream of constant (C) region exons.
For example, to switch from IgM to IgE, recombination loops out the DNA between the Sμ (IgM) and Sε (IgE) regions.
This process is irreversible, as the intervening DNA is excised and lost.
It allows the same VDJ exon to be expressed with a different C-region, changing the antibody isotype without altering antigen specificity
Process Following Activation of Antibody Clones from the Primary Repertoire
Affinity maturation occurs in germinal centres, introducing mutations in the V regions (somatic hypermutation).
Primary repertoire is formed in the bone marrow; a secondary wave of diversification happens in germinal centres.
Mutations in framework regions can disrupt antibody structure → these cells are not selected.
B cells producing higher-affinity antibodies are preferentially selected and expanded.
Somatic Hypermutation → Antibody Diversity
Occurs in germinal centres during immune response.
Introduces point mutations into the heavy chain V-region, especially in CDRs (CDR1 & CDR2 )
Leads to affinity maturation— improves antibody response (higher affinity) overtime via cycles of mutation and selection
(Note): Would be harmful in T cells due to risk of generating self-reactive TCRs—T cells must remain tolerant.