Unit 2
1/98
Earn XP
Description and Tags
Types of Chemical Bonds, Intramolecular Force and Potential Energy, Structure of Ionic Solids, Structure of Metals and Alloys, Lewis Diagrams, Resonance and Formal Charge, VSEPR and Hybridization
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
99 Terms
metallic bonding
sea of electrons act as a buffer between repelling cations, between atoms with similar electronegativities
properties of metallic bonds
good conductors
high MP and BP
malleable
nonpolar covalent bonding
equal/almost equal sharing of electrons, between atoms with similar high electronegativities (same element or C to H)
polar covalent bonding
unequal sharing of electrons, creates dipoles (partial negative/positive poles), between atoms of different high electronegativities
where do the electrons localize closer to in a polar covalent molecule
the atom with higher electronegativity
properties of covalent bonds
poor conductors
when in networks, high MP and BP
when left as molecules, low MP and BP
soft or brittle
ionic bonding
transfer electrons, between atoms with very different electronegativities, attraction between cations and anions
where do the electrons transfer to in an ionic bond
the atom with the higher electronegativity (nonmetal)
properties of ionic bonds
poor conductors when solid
good conductors when liquid
form networks - high MP and BP
brittle
delocalized electrons
electrons that can move around freely
localized electrons
“fixed” electrons restricted to a certain region
intramolecular force
attraction in a bond within the molecule
bond energy/enthalpy
energy released when a bond forms/energy needed to break a bond, refers to covalent bonds
potential energy diagrams
relationship between potential energy (y) and bond length (x)
low potential energy means
more attraction (bond)
what creates higher attraction
smaller atoms, charged atoms, more bonds (double/triple bonds)
lattice energy
amount of energy needed to separate 1 mole of an ionic compound
always mention this when talking about lattice energy
coulomb’s law
coulomb’s law
attraction between two particles is based on charge and distance
which component of attraction is more important
charge
higher lattice energy
higher MP and BP
rxns to form ionic compounds typically
endothermic
rxns to form ionic compounds from gaseous ions are
exothermic because they need to come together to stabilize
lattice structure based on
minimized repulsions and maximized attraction (alternating cation-anion pattern)
alloy
replacing 1 metal atom with another metal atom, usually stronger than metals
types of alloys
substitutional and interstitial
substitutional alloy
consists of 2 metal atoms with similar atomic radii
properties of substitutional alloys
malleable, stronger, retain many metallic properties
interstitial alloys
add smaller atoms to metal atoms, more disruptive effect on metallic bonding
interstitial element
non-metal that covalently bonds to the metal atoms
properties of interstitial alloys
less malleable, significantly stronger
properties of alloys in general
poor conductors, stronger and harder
general process of drawing lewis structures
count valence electrons
determine central atom
LP around terminal atoms
LP on central atom (if applicable)
double/triple bonds (if applicable)
brackets (if applicable)
resonance (if applicable)
how many electrons will fill Be’s valence shell?
4
how many electrons will fill B’s valence shell?
6
which atoms can form an expanded octet
period 3 and under
which atoms can’t form multiple bonds
F, Cl, and H
resonance structures
show all possible lewis structures of a molecule
what happens in a bond orbital-wise
s & p orbitals overlap
sigma bond
s orbitals overlap
pi bond
p orbitals overlap
how many sigma/pi bonds in a single bond
1 sigma
how many sigma/pi bonds in a double bond
1 sigma, 1 pi
how many sigma/pi bonds in a triple bond
1 sigma, 2 pi
hybridization
combines s & p orbitals into a hybrid orbital for bonding, only occurs for the central atom
domain
regions of electrons; bonds and lone pairs
hybridization for 2 domains
sp
hybridization for 3 domains
sp2
hybridization for 4 domains
sp3
formal charge
tells you which structure is correct
formal charge equation
F.C. = #VE - #NBE - 1/2#BE
sum of the formal charges must equal
the charge of the molecule
in a neutral molecule, formal charges of all atoms must optimally be
close to 0
if the formal charge of all atoms cannot be 0, which atom should get the negative charge?
the most electronegative atom
VSEPR theory
molecules arrange to minimize repulsion between electron pairs (will space out as much as possible)
2 domains
linear
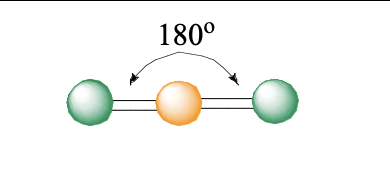
linear
linear bond angle
180
3 domains, 0 lone pairs
trigonal planar
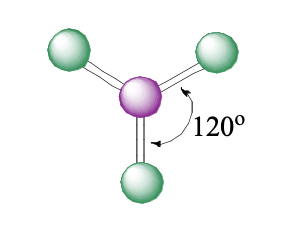
trigonal planar
trigonal planar bond angle
120
3 domains, 1 lone pair
bent
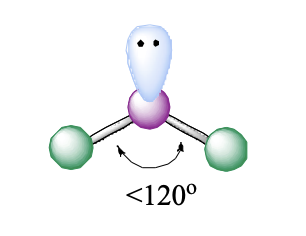
bent
bent (1 LP) bond angle
<120
4 domains, 0 lone pairs
tetrahedral
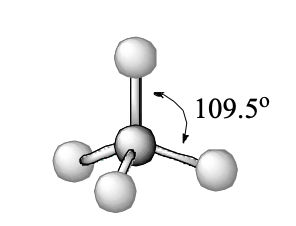
tetrahedral
tetrahedral bond angle
109.5
4 domains, 1 lone pair
trigonal pyramidal
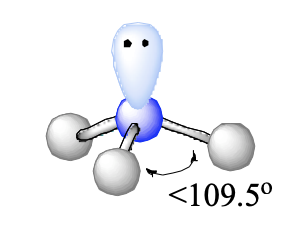
trigonal pyramidal
trigonal pyramidal
<109.5
4 domains, 2 lone pairs
bent
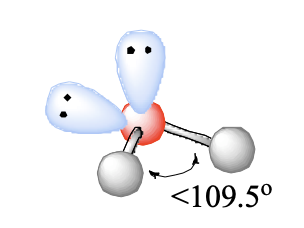
bent
bent (2 LP) bond angle
<109.5
5 domains, 0 lone pairs
trigonal bipyramidal
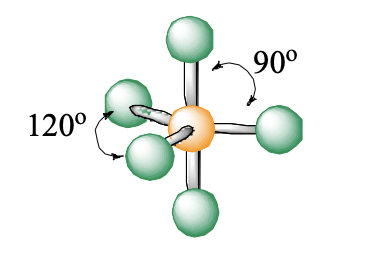
trigonal bipyramidal
trigonal bipyramidal bond angle
90, 120
5 domains, 1 lone pair
see saw
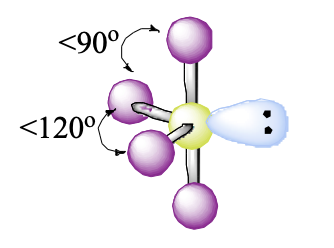
see saw
see saw bond angle
<90, <120
5 domains, 2 lone pairs
t shape
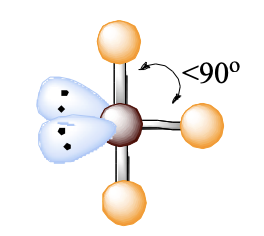
t shape
t shape bond angle
<90
5 domains, 3 lone pairs
linear
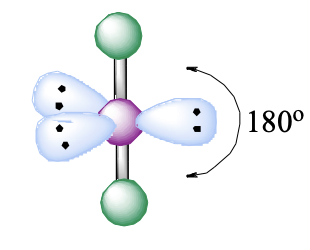
linear
6 domains, 0 lone pairs
octahedral
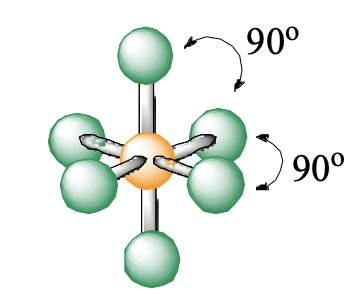
octahedral
octahedral bond angle
90, 90
6 domains, 1 lone pair
square pyramidal
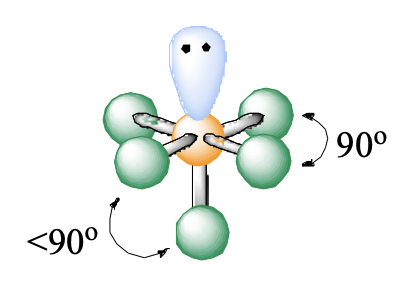
square pyramidal
square pyramidal bond angle
90, <90
6 domains, 2 lone pairs
square planar
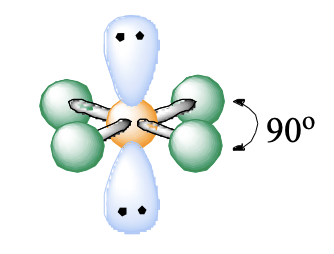
square planar
square planar bond angle
90
how to find electron geometry
count the number of domains; match with molecular geometry with 0 LP
why are the bond angles with lone pairs slightly smaller
LP need more space to move around
nonpolar molecules
symmetrical, even distribution of partial negative/positive charges
polar molecules
asymmetrical, dipole moment
dipole moment
process producing partial charges
bond order
number of bonds / number of domains