GB1-Chapter 8
1/9
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
10 Terms
open system
energy and matter can be transferred between system and its surroundings
1st law of thermodynamics
energy can’t be created or destroyed, but it can be transformed or transferred.
principle of energy conservation
2nd law of thermodynamics
energy is lost as heat during the transformation or transfer
energy transformation/ transfer increases the entropy of the universe- higher disorder
organisms increase the disorder of their surroundings with metabolism- breaking things down to smaller things- more messier
spontaneous processes
happens with no energy input
nonspontaneous process
decreases entropy
requires energy input- because it takes effort to start cleaning, its nonspontaneous and it makes the room cleaner
enzymes
lowers Ea- activation energy so things can happen more easily. Ea activation energy is usually heat.
lowering Ea can happen by realigning/ stretching the substrate.
an enzyme catalyzed reaction can be sped up by increasing the substrate concentration
induced fit
when substrates enters a enzyme, the enzyme changes shape to maximize the catalysis.
saturated enzyme
active site is engaged. if the active site is already saturated, adding more enzyme can speed up reaction time.
cofactors
nonprotein helpers that bind substrates to enzymes
inorganic cofactors- Zu, Fe, Cu
organic cofactors- coenzyme
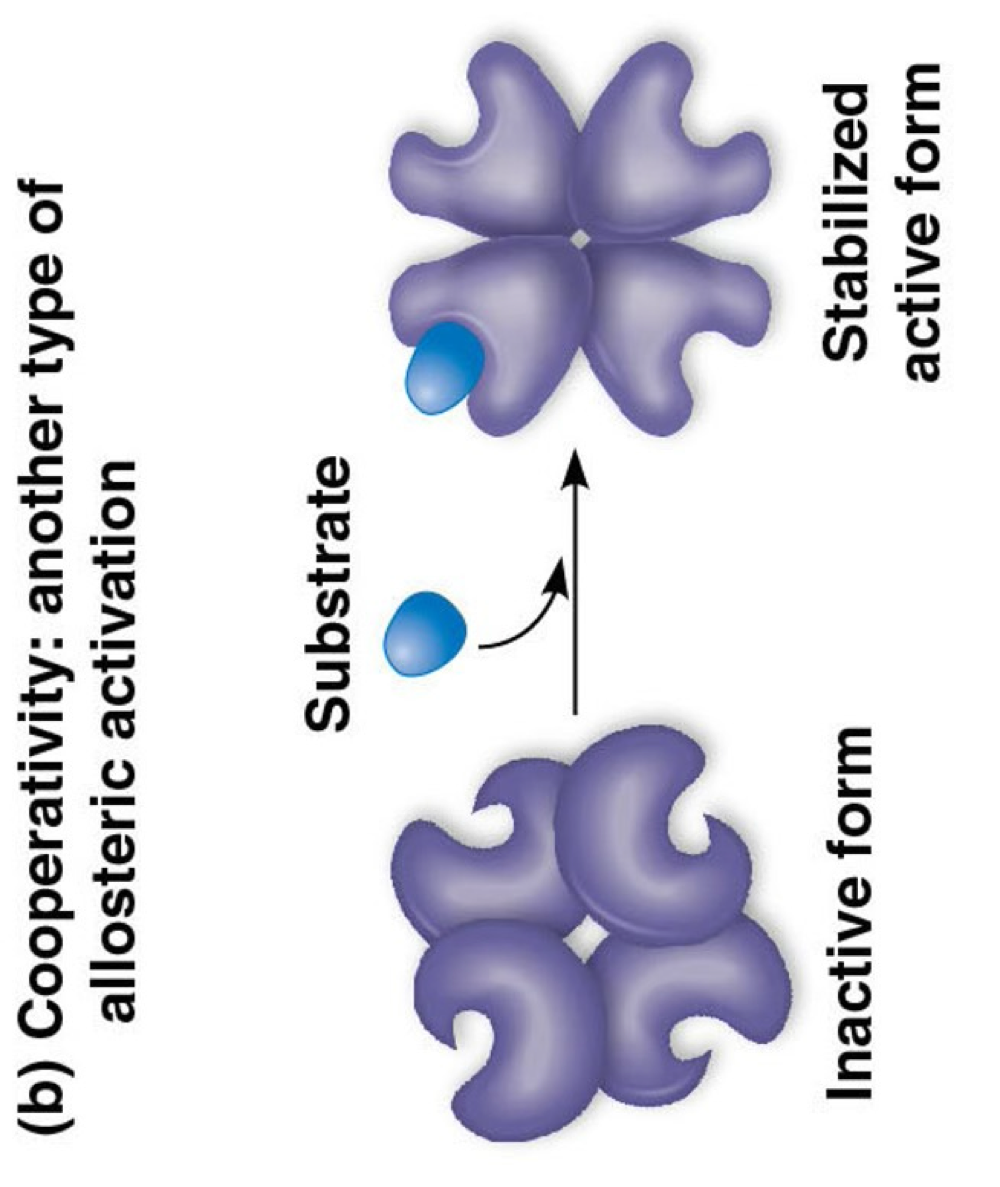
coopertivity
one binding causes shape change- stabilizes all active sites